Which Of The Following Compounds Is Are Chiral
News Leon
Apr 01, 2025 · 5 min read
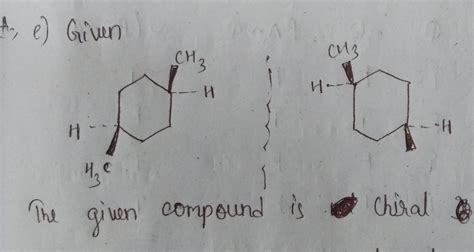
Table of Contents
Which of the Following Compounds is/are Chiral? A Deep Dive into Chirality
Chirality, a fundamental concept in organic chemistry, refers to the handedness of molecules. A chiral molecule is non-superimposable on its mirror image, much like your left and right hands. This property has profound implications in various fields, from pharmaceuticals to materials science. Understanding chirality requires a grasp of several key concepts, including stereocenters, enantiomers, and diastereomers. This article delves into the identification of chiral compounds, exploring different types of molecules and the criteria used to determine their chirality. We’ll tackle the question: which of the following compounds is/are chiral? by examining various structural examples.
Understanding Chirality: The Basics
Before we dive into specific examples, let's solidify our understanding of the fundamental principles of chirality. A molecule is considered chiral if it possesses at least one stereocenter. A stereocenter, often a carbon atom, is a point in a molecule where the interchange of two groups leads to a stereoisomer. The most common type of stereocenter is a chiral carbon atom, also known as an asymmetric carbon. This is a carbon atom bonded to four different groups.
Identifying Chiral Centers: The Four Different Groups Rule
The simplest way to identify a chiral center is to examine each carbon atom in the molecule. If a carbon atom is bonded to four different groups, it's a chiral center, and the molecule is likely chiral (unless there are other elements of symmetry present). If any two groups are identical, the carbon is not a chiral center.
Enantiomers and Diastereomers: Different Types of Stereoisomers
Chiral molecules exist as pairs of enantiomers. Enantiomers are non-superimposable mirror images of each other. They possess identical physical properties (melting point, boiling point, etc.) except for their interaction with plane-polarized light and their behavior in chiral environments. Enantiomers rotate plane-polarized light in opposite directions – one clockwise (+), the other counterclockwise (-).
Diastereomers, on the other hand, are stereoisomers that are not mirror images of each other. They arise when a molecule has more than one chiral center. Diastereomers have different physical properties.
Analyzing Specific Compounds for Chirality
Let's now apply our understanding to determine the chirality of several hypothetical compounds. We'll analyze each molecule systematically, identifying potential chiral centers and assessing their impact on the overall chirality of the molecule.
Example 1: 2-Bromobutane
CH3-CHBr-CH2-CH3
This molecule possesses a chiral carbon atom (the second carbon). It's bonded to four different groups: a bromine atom (Br), a methyl group (CH3), an ethyl group (CH2CH3), and a hydrogen atom (H). Therefore, 2-bromobutane is chiral. It exists as a pair of enantiomers.
Example 2: 2,3-Dibromobutane
CH3-CHBr-CHBr-CH3
This molecule presents a slightly more complex scenario. It has two chiral centers (the second and third carbons). However, we need to consider whether the molecule as a whole is chiral. In this case, the molecule is achiral. It is a meso compound. A meso compound is a molecule with multiple chiral centers but possesses an internal plane of symmetry, making it superimposable on its mirror image.
Example 3: 1-Bromobutane
CH3-CH2-CH2-CH2Br
This molecule contains no chiral centers. The first carbon is bonded to three hydrogens and one ethyl group. The second, third, and fourth carbon are bonded to at least two identical groups. Therefore, 1-bromobutane is achiral.
Example 4: 2-Chloropropane
CH3-CHCl-CH3
This molecule has a carbon atom attached to a chlorine atom, a methyl group, and two hydrogen atoms. Since two groups are identical (hydrogens), this carbon atom is not a stereocenter. Therefore, 2-chloropropane is achiral.
Example 5: 2,3-Dichlorobutane
This molecule presents a more nuanced situation. It has two chiral centers, similar to 2,3-dibromobutane. However, unlike 2,3-dibromobutane, it does not possess a plane of symmetry. Thus, 2,3-dichlorobutane is chiral. It exists as a pair of enantiomers, plus two diastereomers (the meso compound is not a diastereomer because it is identical to its enantiomer).
Example 6: 1,2-Dibromopropane
CH3-CHBr-CH2Br
This molecule has one chiral center (the second carbon), bonded to a methyl group, a hydrogen, a bromine atom, and a bromomethyl group. Therefore, 1,2-dibromopropane is chiral.
Example 7: 1,3-Dibromopropane
CH2Br-CH2-CH2Br
No chiral centers are present in this molecule. It is achiral.
Example 8: 1,2-Dichloroethane
CH2Cl-CH2Cl
This molecule is achiral. It does not have any chiral centers.
Chirality and its Importance
The chirality of molecules has significant implications across various scientific disciplines:
-
Pharmacology: Enantiomers of a drug can have vastly different biological activities. One enantiomer might be effective, while the other is inactive or even toxic. This underscores the importance of producing and administering drugs in their pure enantiomeric forms.
-
Materials Science: Chiral molecules can be used to create materials with specific optical properties, leading to applications in liquid crystals and other advanced materials.
-
Biochemistry: Many biologically important molecules, such as amino acids and sugars, are chiral. Their specific chirality plays a crucial role in their biological function and interactions.
Advanced Concepts and Considerations
While the "four different groups" rule is a helpful starting point, it's important to note that other factors can influence chirality:
-
Axial Chirality: Molecules can be chiral due to the presence of axial chirality, where the chirality is associated with restricted rotation around a bond, resulting in non-superimposable conformations. Allenes and biphenyls are common examples.
-
Planar Chirality: Certain molecules possess chirality due to restricted rotation around a bond, leading to non-superimposable conformations.
Conclusion: Determining Chirality – A Systematic Approach
Determining whether a compound is chiral requires a systematic approach. Carefully examine each carbon atom in the molecule. If a carbon is bonded to four different groups, it's a chiral center, and the molecule is likely chiral (although the presence of internal symmetry needs to be considered). Understanding the concepts of enantiomers, diastereomers, and meso compounds is crucial for a complete understanding of chirality. The implications of chirality extend across various fields, highlighting its significance in both fundamental and applied research. Remember to always consider other forms of chirality beyond the simple tetrahedral carbon center. Practice with various molecular structures is key to mastering chirality determination.
Latest Posts
Latest Posts
-
Does Reactivity Increase Down A Group
Apr 02, 2025
-
How Many Vertices Does Octagon Have
Apr 02, 2025
-
Reaction Of Ammonia And Sulfuric Acid
Apr 02, 2025
-
What Type Of Triangle Is Shown Below
Apr 02, 2025
-
Which Of The Following Pairs Are Mismatched
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Compounds Is Are Chiral . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
