Which Lewis Electron Dot Diagram Is Correct For Co2
News Leon
Mar 31, 2025 · 5 min read
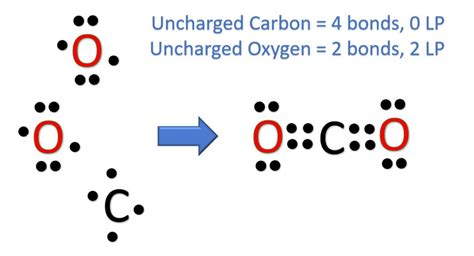
Table of Contents
Which Lewis Electron Dot Diagram is Correct for CO₂? A Deep Dive into Molecular Structure
Determining the correct Lewis electron dot diagram for a molecule is crucial for understanding its bonding, geometry, and properties. Carbon dioxide (CO₂) provides a compelling case study, as its seemingly simple structure can lead to confusion if the rules of Lewis structures aren't meticulously followed. This article will explore the various possible Lewis structures for CO₂, analyze their validity based on formal charges and octet rule satisfaction, and ultimately identify the most accurate representation.
Understanding Lewis Structures and the Octet Rule
Before diving into the specific case of CO₂, let's briefly review the fundamental principles of drawing Lewis structures. The goal is to represent the valence electrons of atoms in a molecule, illustrating how they participate in bonding to achieve stability. The octet rule, a cornerstone of Lewis structure theory, states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons (similar to the electron configuration of noble gases). There are exceptions to the octet rule, but it serves as a valuable guideline in most cases.
Steps to Draw a Lewis Structure:
-
Count valence electrons: Add up the valence electrons of all atoms in the molecule. Carbon has 4 valence electrons, and each oxygen atom has 6, giving a total of 16 valence electrons for CO₂.
-
Identify the central atom: The least electronegative atom is usually the central atom. In CO₂, carbon is less electronegative than oxygen and thus sits in the center.
-
Connect atoms with single bonds: Place single bonds (represented by lines) between the central atom and each surrounding atom. This uses 4 electrons (2 bonds x 2 electrons/bond).
-
Distribute remaining electrons: Place the remaining electrons (16 - 4 = 12 electrons) as lone pairs around the outer atoms to satisfy the octet rule (or duet rule for hydrogen).
-
Satisfy the octet rule for the central atom: If the central atom doesn't have an octet, form double or triple bonds by moving lone pairs from outer atoms to form additional bonds with the central atom.
Possible Lewis Structures for CO₂ and Their Evaluation
Now let's apply these steps to CO₂ and examine the resulting structures:
Structure 1: Single Bonds
O-C-O
With this structure, we've used 4 electrons for the single bonds. We have 12 electrons remaining. Placing these as lone pairs on the oxygen atoms gives each oxygen an octet:
:O-C-O:
However, carbon only has 4 electrons, failing to satisfy the octet rule. This structure is incorrect.
Structure 2: One Double Bond, One Single Bond
O=C-O
This structure uses 6 electrons for one double bond and 2 electrons for the single bond, leaving 8 electrons to be placed as lone pairs. Placing these gives one oxygen an octet and the other seven electrons, which is also incorrect. The carbon atom also only has 6 electrons. This structure is also incorrect.
Structure 3: Two Double Bonds
O=C=O
This structure uses 8 electrons (4 bonds * 2 electrons/bond) leaving 8 electrons (16 - 8 = 8) to be distributed as lone pairs. Placing four lone pairs on each oxygen atom results in a structure where both oxygen and carbon atoms satisfy the octet rule:
:O=C=O:
This structure is correct because it satisfies the octet rule for all atoms and minimizes formal charges.
Formal Charges and Their Significance
Formal charge is a useful tool for evaluating the validity of Lewis structures. It represents the difference between the number of valence electrons in an isolated atom and the number of electrons assigned to that atom in the Lewis structure. The formula for formal charge is:
Formal Charge = (Valence electrons) - (Non-bonding electrons) - (1/2 * Bonding electrons)
For Structure 3 (the correct structure), the formal charge calculation for each atom is:
- Carbon: 4 (valence) - 0 (non-bonding) - (1/2 * 8 bonding) = 0
- Oxygen (each): 6 (valence) - 4 (non-bonding) - (1/2 * 4 bonding) = 0
A Lewis structure with formal charges closest to zero is generally preferred. Structures with significant positive or negative formal charges are less stable.
Resonance Structures in CO₂
While the double-bonded structure (Structure 3) is the most accurate representation of CO₂, it's important to acknowledge the concept of resonance. Resonance describes the delocalization of electrons across multiple bonds. In the case of CO₂, we can draw two equivalent resonance structures:
:O=C-O: <--> :O-C=O:
These two structures are not distinct molecules; they represent the same molecule with electron delocalization between the two C=O bonds. The true structure is a hybrid of these two resonance structures, with the bond order of each C-O bond being 2 (a bond order of 2 represents a double bond).
VSEPR Theory and the Linear Geometry of CO₂
The Lewis structure accurately predicts the molecular geometry of CO₂ using VSEPR (Valence Shell Electron Pair Repulsion) theory. The carbon atom has two bonding pairs and no lone pairs, leading to a linear geometry. This linear arrangement minimizes electron-electron repulsion and is consistent with experimental observations.
Conclusion: The Correct Lewis Structure for CO₂
After comprehensively examining various possible Lewis structures and applying the octet rule, formal charge analysis, and VSEPR theory, the most accurate Lewis structure for CO₂ is the one with two double bonds:
:O=C=O:
This structure correctly represents the octet rule satisfaction for all atoms, minimizes formal charges, and accurately predicts the linear geometry of the molecule. While resonance structures need to be considered to fully understand the electron distribution, the double-bonded structure provides the best single representation of the molecule's bonding. Understanding the nuances of Lewis structures, formal charges, and resonance is essential for accurately depicting molecular structures and properties. The application of these principles to CO₂ not only clarifies its bonding but also serves as an excellent example for understanding more complex molecules. This detailed analysis highlights the importance of following the rules of Lewis structures rigorously, leading to a deeper understanding of molecular bonding and geometry. Remember, the accurate representation of molecular structure is crucial for understanding the various properties and interactions within chemical systems.
Latest Posts
Latest Posts
-
Which Of The Following Temperatures Is The Coldest
Apr 01, 2025
-
A Short Term Unsecured Promissory Note Issued By A Company Is
Apr 01, 2025
-
Adjacent Angles Whose Sum In 180 Degrees
Apr 01, 2025
-
Lewis Dot Structure For Magnesium Chloride
Apr 01, 2025
-
A Group Of Related Records Is Called A Table
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Lewis Electron Dot Diagram Is Correct For Co2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
