Whether A Molecule Can Cross The Plasma Membrane Depends Upon
News Leon
Mar 19, 2025 · 5 min read
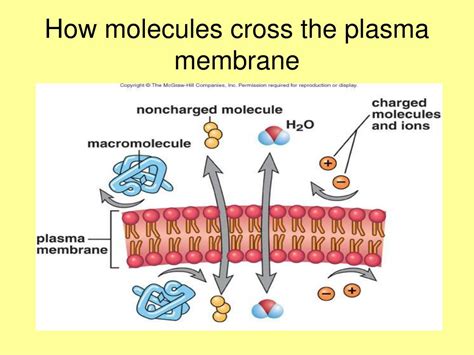
Table of Contents
Whether a Molecule Can Cross the Plasma Membrane Depends Upon…
The plasma membrane, a selectively permeable barrier, is crucial for maintaining cellular homeostasis. Its ability to regulate the passage of molecules is paramount to cell survival and function. But what exactly dictates whether a molecule can traverse this critical boundary? The answer is multifaceted, depending on a complex interplay of factors related to the molecule itself and the membrane's structure. This article delves into the intricate details of how a molecule's properties influence its ability to cross the plasma membrane.
The Nature of the Plasma Membrane: A Dynamic Barrier
Before exploring the factors influencing molecular passage, let's briefly review the plasma membrane's structure. It's a fluid mosaic model, primarily composed of a phospholipid bilayer. These phospholipids are amphipathic molecules, possessing both hydrophilic (water-loving) heads and hydrophobic (water-fearing) tails. This arrangement creates a hydrophobic core sandwiched between two hydrophilic surfaces. Embedded within this bilayer are various proteins, cholesterol molecules, and glycolipids, all contributing to the membrane's selective permeability.
Key Components Affecting Permeability:
-
Phospholipid Bilayer: The fundamental structure dictates the initial barrier. Small, nonpolar molecules can readily diffuse across this hydrophobic core. However, larger or polar molecules face significant resistance.
-
Membrane Proteins: These integral and peripheral proteins play a crucial role in facilitating the transport of specific molecules. Some act as channels, allowing passive movement down a concentration gradient. Others function as carriers, actively transporting molecules against their concentration gradient, requiring energy.
-
Cholesterol: This molecule modulates membrane fluidity, affecting the permeability of the membrane to different molecules.
Factors Determining Molecular Passage:
Several factors determine whether a molecule can successfully negotiate the plasma membrane:
1. Molecular Size and Shape:
Smaller molecules generally diffuse across the membrane more easily than larger ones. This is because smaller molecules can more readily navigate the spaces between phospholipid molecules within the hydrophobic core. Shape also plays a role; a molecule's conformation can influence its ability to interact with the membrane components. Linear molecules might pass more easily than highly branched ones. Size and shape are critical factors influencing passive diffusion.
2. Lipid Solubility:
Lipid solubility is arguably the most crucial factor. Molecules that are highly soluble in lipids (lipophilic or hydrophobic) can readily dissolve into the hydrophobic core of the membrane and diffuse across. Conversely, molecules that are hydrophilic (water-loving) struggle to penetrate this hydrophobic barrier. This explains why many small, nonpolar molecules like oxygen (O2) and carbon dioxide (CO2) readily diffuse across the membrane, while polar molecules like glucose require facilitated transport.
3. Polarity and Charge:
Polar molecules, possessing an uneven distribution of charge, encounter difficulty crossing the hydrophobic core. The interaction between the polar molecule and the hydrophobic environment is energetically unfavorable. Charged molecules (ions) face an even greater challenge. Their strong interaction with water molecules makes it energetically expensive for them to enter the hydrophobic region. Therefore, polar and charged molecules generally rely on membrane proteins for transport.
4. Concentration Gradient:
The concentration gradient across the membrane significantly impacts passive transport. Molecules move passively from regions of high concentration to regions of low concentration, following the concentration gradient. This process requires no energy input. However, the rate of passive diffusion is directly proportional to the concentration gradient. A steeper gradient means faster diffusion. This is particularly relevant for small, nonpolar molecules.
5. Membrane Potential:
The membrane potential, the electrical potential difference across the membrane, influences the transport of charged molecules (ions). The membrane potential creates an electrochemical gradient, combining the effects of the concentration gradient and the electrical gradient. For example, positively charged ions (cations) are attracted to the negatively charged interior of the cell, while negatively charged ions (anions) are repelled. This electrical driving force can either facilitate or hinder the transport of ions.
6. Membrane Protein Mediated Transport:
Many molecules, particularly larger, polar, or charged molecules, cannot passively diffuse across the membrane. Instead, they rely on membrane proteins for transport. There are two main types:
-
Facilitated Diffusion: This process utilizes membrane proteins (channels or carriers) to facilitate the passive movement of molecules down their concentration gradient. No energy is required. Channels form hydrophilic pores that allow specific molecules to pass through. Carriers bind to the molecule and undergo conformational changes to transport it across the membrane.
-
Active Transport: This process uses membrane proteins to move molecules against their concentration gradient, requiring energy, usually in the form of ATP. Active transport allows cells to maintain concentration gradients that differ significantly from the external environment. Examples include the sodium-potassium pump, essential for maintaining nerve impulse transmission.
Specific Examples:
Let's illustrate these principles with specific examples:
-
Oxygen (O2): A small, nonpolar molecule; it readily diffuses across the membrane by passive diffusion.
-
Carbon Dioxide (CO2): Similar to oxygen, its small size and nonpolar nature allow for passive diffusion.
-
Glucose: A large, polar molecule; it requires facilitated diffusion via glucose transporters (GLUTs).
-
Sodium Ions (Na+): A charged ion; its transport is primarily mediated by active transport via the sodium-potassium pump.
-
Water (H2O): Although polar, its small size allows for a significant degree of passive diffusion, particularly through aquaporins (water channels).
-
Steroid Hormones: These lipid-soluble hormones readily diffuse across the plasma membrane.
Conclusion:
Whether a molecule can cross the plasma membrane depends on a complex interplay of factors, primarily its size, shape, lipid solubility, polarity, and charge. While small, nonpolar molecules can passively diffuse across the lipid bilayer, larger, polar, or charged molecules often require the assistance of membrane proteins, either through facilitated diffusion or active transport. Understanding these principles is fundamental to comprehending cellular function, signaling pathways, and the maintenance of cellular homeostasis. The selective permeability of the plasma membrane, governed by these factors, is crucial for life itself. Further research into these complex interactions continues to unveil the intricate mechanisms governing molecular transport across this fundamental cellular barrier. The dynamic interplay between the molecule's properties and the membrane's structure continues to be a fascinating area of ongoing biological study. Future research promises to further elucidate the intricacies of this vital cellular process.
Latest Posts
Latest Posts
-
Is Supporting Combustion A Physical Or Chemical Property
Mar 19, 2025
-
What Class Lever Is A Wheelbarrow
Mar 19, 2025
-
The Sum Of Two Polynomials Is 10a2
Mar 19, 2025
-
Which Of The Following Events Occur During Anaphase I
Mar 19, 2025
-
How Did Hoover React To The Bonus Army
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Whether A Molecule Can Cross The Plasma Membrane Depends Upon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
