When A Cell Is Placed In A Hypertonic Solution
News Leon
Mar 28, 2025 · 5 min read
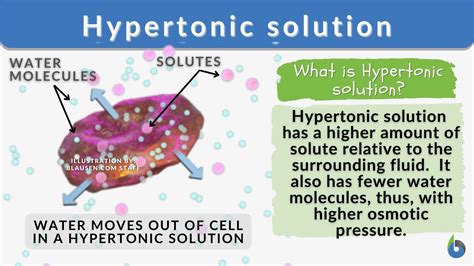
Table of Contents
When a Cell is Placed in a Hypertonic Solution: Osmosis and its Effects
Understanding how cells react to different environments is fundamental to biology. A key concept in this understanding is osmosis, the movement of water across a selectively permeable membrane from an area of high water concentration to an area of low water concentration. This article delves into the fascinating world of osmosis, focusing specifically on what happens when a cell is placed in a hypertonic solution.
What is a Hypertonic Solution?
Before diving into the effects on a cell, we need to define what a hypertonic solution is. A hypertonic solution is one that has a higher solute concentration compared to another solution, typically the cell's cytoplasm. "Hyper" implies "above" or "greater than," referring to the solute concentration. The solute can be anything dissolved in the solvent (usually water), including salts, sugars, or other molecules. The key takeaway is that a hypertonic solution has a lower water concentration than the solution it's being compared to.
Osmosis: The Driving Force
Osmosis is a passive transport process, meaning it doesn't require energy input from the cell. Water molecules, being small and uncharged, can freely move across the cell membrane through specialized channels called aquaporins. The driving force behind osmosis is the difference in water potential between the two solutions separated by the membrane. Water always moves from an area of higher water potential (less solute, more water) to an area of lower water potential (more solute, less water).
Water Potential: A Closer Look
Water potential is a measure of the tendency of water to move from one area to another. It's affected by two main factors:
- Solute potential: This is the effect of dissolved solutes on water potential. A higher solute concentration lowers the water potential.
- Pressure potential: This is the effect of physical pressure on water potential. Positive pressure (like turgor pressure in a plant cell) increases water potential, while negative pressure (like tension in a plant's xylem) decreases it.
In a hypertonic solution, the solute potential is lower (more negative) than inside the cell, resulting in a net movement of water out of the cell.
Effects on Different Cell Types
The effects of placing a cell in a hypertonic solution vary depending on the type of cell:
Animal Cells: Crenation
When an animal cell is placed in a hypertonic solution, water flows out of the cell through osmosis, causing the cell to shrink. This process is called crenation. As water leaves, the cell membrane pulls away from the cell wall (if present), and the cell becomes increasingly dehydrated. Severe crenation can lead to cell death.
Plant Cells: Plasmolysis
Plant cells have a rigid cell wall surrounding the cell membrane. When a plant cell is placed in a hypertonic solution, water also moves out of the cell by osmosis. However, the cell wall prevents the cell from completely collapsing. Instead, the cell membrane pulls away from the cell wall, a process known as plasmolysis. The space between the cell membrane and the cell wall fills with the hypertonic solution. Plasmolysis causes the plant cell to lose its turgor pressure, becoming flaccid and wilted.
Bacterial Cells: Plasmolysis (Similar to Plant Cells)
Bacterial cells, like plant cells, also possess a cell wall, albeit with a different structure. When subjected to a hypertonic environment, similar to plant cells, bacterial cells undergo plasmolysis, losing water and shrinking. This process can affect their metabolic activities and viability.
The Importance of Maintaining Osmoregulation
The ability of cells and organisms to maintain a stable internal environment, despite external changes, is known as osmoregulation. This is crucial for survival. Cells have evolved various mechanisms to cope with changes in osmotic pressure, including:
- Contractile vacuoles: Found in some single-celled organisms like Paramecium, these organelles actively pump excess water out of the cell to prevent it from bursting in hypotonic environments. This is less critical in hypertonic situations where the problem is water loss.
- Ion pumps: Cells use energy to actively transport ions in and out, influencing the osmotic balance.
- Cell wall: As discussed, the rigid cell wall in plants and bacteria protects cells from shrinking excessively in hypertonic solutions.
Real-World Examples of Hypertonic Solutions and their Effects
Understanding hypertonic solutions has numerous applications in various fields:
- Food preservation: High concentrations of salt or sugar create hypertonic environments that inhibit microbial growth, preserving food. Think of pickled vegetables or jams.
- Medicine: Hypertonic solutions are used in intravenous therapy to treat dehydration or edema (fluid buildup). They draw water from tissues into the bloodstream.
- Agriculture: The appropriate soil salinity is vital for plant growth. Excessive salt can create hypertonic conditions, leading to plasmolysis and stunted plant growth.
Experimental Demonstrations
Observing the effects of a hypertonic solution on cells is a common biology experiment. Using simple materials such as Elodea leaves (a common aquatic plant) and salt water, one can easily demonstrate plasmolysis. By observing the changes in the plant cells under a microscope, the effects of water loss and the shrinkage of the cytoplasm become visually apparent. Similar experiments using cheek cells and a salt solution can demonstrate crenation in animal cells.
Beyond the Basics: Further Considerations
The discussion above simplifies the complex interactions between cells and their surroundings. Several additional factors can influence the outcome when a cell is placed in a hypertonic solution:
- Cell membrane permeability: The permeability of the cell membrane to different solutes affects the overall osmotic pressure.
- Solution composition: The specific type of solute in the hypertonic solution can influence the rate and extent of water movement.
- Cell size and shape: Larger cells might have a larger surface area to volume ratio, potentially affecting their response to osmotic stress.
Conclusion
The response of a cell to a hypertonic solution, whether crenation or plasmolysis, is a fundamental consequence of osmosis. Understanding this process is crucial for comprehending various biological phenomena, ranging from cell survival to food preservation techniques and medical applications. The ability of organisms to regulate their internal water balance through osmoregulation is a testament to the elegance and complexity of life's processes. Further research into these complex interactions continues to reveal the intricate workings of cellular life and its adaptability to various environments. The interplay between osmosis, solute concentration, and cellular structure offers a rich field of study with implications across diverse scientific disciplines.
Latest Posts
Latest Posts
-
How Do You Write 1 00 As A Percentage
Mar 31, 2025
-
Draw The Bohr Model For Aluminum
Mar 31, 2025
-
Which Particles Are Transferred During A Redox Reaction
Mar 31, 2025
-
Authorization Letter To Act On My Behalf
Mar 31, 2025
-
Which Of The Following Are Cash Outflows From Financing Activities
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about When A Cell Is Placed In A Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
