Which Particles Are Transferred During A Redox Reaction
News Leon
Mar 31, 2025 · 7 min read
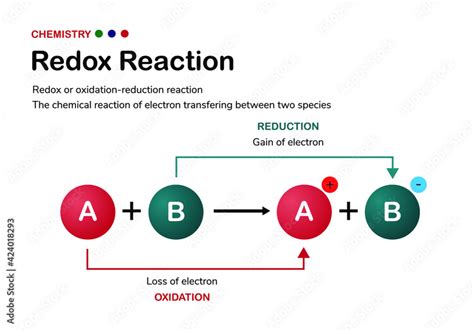
Table of Contents
Which Particles Are Transferred During a Redox Reaction? A Deep Dive into Electron Transfer
Redox reactions, short for reduction-oxidation reactions, are fundamental processes in chemistry and biology. They underpin a vast array of phenomena, from rusting and combustion to photosynthesis and cellular respiration. At the heart of every redox reaction lies the transfer of electrons. Understanding which particles are transferred and how this transfer occurs is crucial to grasping the essence of these reactions. This article will explore the intricacies of electron transfer in redox reactions, delving into the specifics of particle movement and the implications for various chemical and biological systems.
The Fundamentals of Redox Reactions: Oxidation and Reduction
Before we dive into the specifics of particle transfer, let's establish a clear understanding of oxidation and reduction. These two processes are always coupled; one cannot occur without the other.
-
Oxidation: This involves the loss of electrons by a species. The species undergoing oxidation is called the reducing agent because it donates electrons to another species, causing its reduction. A common mnemonic to remember oxidation is "OIL RIG" – Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons).
-
Reduction: This involves the gain of electrons by a species. The species undergoing reduction is called the oxidizing agent because it accepts electrons from another species, causing its oxidation.
The key to recognizing a redox reaction is to look for changes in oxidation states (or oxidation numbers). An increase in oxidation state signifies oxidation, while a decrease signifies reduction.
Electrons as the Primary Transferred Particles
The most fundamental particle transferred during a redox reaction is the electron. Electrons are negatively charged subatomic particles that orbit the nucleus of an atom. Their transfer is the driving force behind the entire redox process. When an atom or molecule loses electrons (oxidation), it becomes more positively charged (or less negatively charged). Conversely, when an atom or molecule gains electrons (reduction), it becomes more negatively charged (or less positively charged).
This electron transfer can be visualized as a flow of charge, with electrons moving from the reducing agent (which loses electrons and is oxidized) to the oxidizing agent (which gains electrons and is reduced). This transfer doesn't necessarily involve direct physical contact between the reacting species; it can occur through various mechanisms, including:
-
Direct electron transfer: In some cases, electrons transfer directly from the reducing agent to the oxidizing agent. This often occurs in solutions where the reactants are in close proximity.
-
Electron transfer through a mediator: Other reactions involve electron transfer through a mediator, a molecule that accepts electrons from the reducing agent and then donates them to the oxidizing agent. This is common in biological systems where enzymes often act as mediators in redox reactions.
-
Electron transfer through a solid electrode: In electrochemical cells, electrons are transferred through an external circuit from the anode (where oxidation occurs) to the cathode (where reduction occurs).
Beyond Electrons: The Role of Protons and Other Ions
While electrons are the primary particles transferred, other particles can also be involved, particularly in reactions involving hydrogen. Many redox reactions, especially those in aqueous solutions, involve the transfer of protons (H⁺). The transfer of protons is often coupled with the transfer of electrons.
Consider the reduction of a proton to form hydrogen gas:
2H⁺ + 2e⁻ → H₂
Here, two protons gain two electrons to form a molecule of hydrogen gas. This reaction is a classic example of a reduction, and it showcases the simultaneous transfer of both protons and electrons. Note that in this instance, the protons are not inherently transferred as part of a redox process but rather participate in the reduction of the protons themselves.
In biological systems, the transfer of hydride ions (H⁻), which consists of a proton and two electrons, is also common. This often occurs in enzyme-catalyzed reactions involving coenzymes like NAD⁺/NADH and FAD/FADH₂, which are crucial for cellular respiration. Here, the hydride ion acts as a single entity in the transfer process rather than a proton and electrons transferred separately.
Furthermore, other ions can participate indirectly in redox reactions. For example, in many metal-ion redox reactions, the metal ion changes its oxidation state by gaining or losing electrons. The counter-ions associated with these metal ions remain present, but they don't directly participate in the electron transfer process itself.
Examples of Redox Reactions and Particle Transfer
Let's examine some specific examples to illustrate the transfer of particles during redox reactions:
1. Rusting of Iron:
The rusting of iron (Fe) is a classic example of a redox reaction. Iron reacts with oxygen (O₂) and water (H₂O) to form iron(III) oxide (Fe₂O₃), commonly known as rust.
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
In this reaction, iron atoms lose electrons (oxidation) to form Fe³⁺ ions, while oxygen atoms gain electrons (reduction) to form oxide ions (O²⁻). The overall process involves the transfer of electrons from iron to oxygen.
2. Combustion of Methane:
The combustion of methane (CH₄) is another example of a redox reaction. Methane reacts with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O).
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
In this reaction, carbon in methane is oxidized (loses electrons) to form carbon dioxide, while oxygen is reduced (gains electrons) to form water. This reaction involves the transfer of electrons from carbon to oxygen, often accompanied by proton transfer within the water molecules formed.
3. Photosynthesis:
Photosynthesis is a complex biological redox reaction that converts light energy into chemical energy. During photosynthesis, water is oxidized to produce oxygen, while carbon dioxide is reduced to produce glucose.
6CO₂(g) + 6H₂O(l) → C₆H₁₂O₆(aq) + 6O₂(g)
This reaction involves the transfer of electrons from water to carbon dioxide, with protons also participating in the overall process. This sophisticated reaction utilizes various electron carriers and enzyme systems to facilitate the complex electron and proton transfer.
4. Cellular Respiration:
Cellular respiration is the reverse of photosynthesis, where glucose is oxidized to produce carbon dioxide and water, releasing energy in the process.
C₆H₁₂O₆(aq) + 6O₂(g) → 6CO₂(g) + 6H₂O(l)
This reaction involves the transfer of electrons from glucose to oxygen, generating ATP (adenosine triphosphate), the cell's primary energy currency. This intricate process also involves multiple steps with electron carriers like NADH and FADH₂, facilitating electron and proton transfer within the electron transport chain.
Implications of Electron Transfer in Redox Reactions
The transfer of electrons during redox reactions has profound implications across numerous fields:
-
Corrosion and Protection of Metals: Understanding redox reactions is crucial in preventing corrosion of metals, a process where metals are oxidized and lose their structural integrity. Protective coatings and cathodic protection methods are based on principles of redox reactions to hinder the oxidation process.
-
Energy Production and Storage: Redox reactions are fundamental to energy production in fuel cells and batteries. These devices harness the energy released during redox reactions to generate electricity.
-
Biochemistry and Metabolism: Redox reactions are essential for biological processes such as respiration, photosynthesis, and nitrogen fixation. The transfer of electrons drives energy production and the synthesis of essential biomolecules.
-
Environmental Chemistry: Redox reactions play a crucial role in environmental processes, including the oxidation and reduction of pollutants, the cycling of nutrients, and the formation of minerals.
-
Industrial Processes: Many industrial processes rely on redox reactions, including the extraction of metals from ores, the synthesis of chemicals, and the treatment of wastewater.
Conclusion
Redox reactions are ubiquitous in nature and technology. At their core, these reactions involve the transfer of electrons, often coupled with the transfer of protons or other ions. This fundamental electron transfer underlies a wide spectrum of processes, from the rusting of iron to the complex biochemical reactions that sustain life. A deep understanding of redox reactions, specifically the particles transferred and the mechanisms involved, is essential for advancements in various scientific and technological fields. Further research continues to unravel the intricacies of electron transfer and its profound impact on our world.
Latest Posts
Latest Posts
-
Select The Four Statements About Plasmodium That Are True
Apr 02, 2025
-
Greatest Common Factor Of 36 And 20
Apr 02, 2025
-
What Is The Antonym Of Urban
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Particles Are Transferred During A Redox Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
