Draw The Bohr Model For Aluminum
News Leon
Mar 31, 2025 · 6 min read
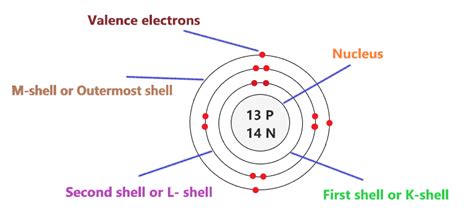
Table of Contents
Drawing the Bohr Model for Aluminum: A Comprehensive Guide
The Bohr model, while a simplified representation of atomic structure, provides a valuable visual tool for understanding the arrangement of electrons in an atom. This article will guide you through the process of drawing the Bohr model for aluminum (Al), explaining the underlying principles and providing a step-by-step approach. We’ll also explore the limitations of the Bohr model and delve into more advanced concepts.
Understanding the Basics of the Bohr Model
Before we dive into drawing the aluminum Bohr model, let's refresh our understanding of the fundamental principles:
- Nucleus: At the center of the atom lies the nucleus, containing protons (positively charged) and neutrons (neutrally charged). The number of protons defines the element's atomic number.
- Electron Shells (Energy Levels): Electrons orbit the nucleus in specific energy levels or shells. These shells are represented by concentric circles around the nucleus. The closer a shell is to the nucleus, the lower its energy level.
- Electron Capacity: Each shell can hold a maximum number of electrons. The first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold a maximum of 8 electrons, and the third shell (n=3) can hold a maximum of 18 electrons. However, for simplicity, the Bohr model often depicts the third shell's maximum capacity as 8 electrons.
Determining the Number of Protons, Neutrons, and Electrons in Aluminum
To draw the Bohr model for aluminum, we first need to determine the number of protons, neutrons, and electrons it possesses. We can find this information using the periodic table:
- Atomic Number: Aluminum's atomic number is 13. This means it has 13 protons.
- Number of Electrons: In a neutral atom, the number of electrons equals the number of protons. Therefore, aluminum has 13 electrons.
- Mass Number and Neutrons: Aluminum's most common isotope has a mass number of 27. The mass number is the sum of protons and neutrons. To find the number of neutrons, subtract the atomic number (protons) from the mass number: 27 - 13 = 14 neutrons.
Step-by-Step Guide to Drawing the Bohr Model for Aluminum
Now, let's construct the Bohr model for aluminum:
-
Draw the Nucleus: Start by drawing a large circle in the center of your paper to represent the nucleus. Inside the nucleus, write "13p<sup>+</sup>" (13 protons) and "14n<sup>0</sup>" (14 neutrons).
-
First Electron Shell (n=1): Draw a smaller circle around the nucleus. This represents the first electron shell. Aluminum's first shell can hold a maximum of 2 electrons. Place two small dots or crosses within this first shell to represent these electrons.
-
Second Electron Shell (n=2): Draw a larger circle around the first shell. This is the second electron shell, which can hold a maximum of 8 electrons. Place eight small dots or crosses within this second shell to represent these electrons.
-
Third Electron Shell (n=3): Draw an even larger circle around the second shell. This is the third electron shell. Aluminum has 13 electrons, and we've already placed 10 (2+8) in the first two shells. Therefore, the remaining 3 electrons go into the third shell. Place three small dots or crosses within this third shell.
Your completed Bohr model should show:
- A nucleus containing 13 protons and 14 neutrons.
- A first shell with 2 electrons.
- A second shell with 8 electrons.
- A third shell with 3 electrons.
Visual Representation (Text-Based)
While a visual diagram is ideal, we can represent the Bohr model textually:
(3e-)
-------
| |
| (8e-) | <-- Third Shell (n=3)
| |
-------
-------
| |
| (2e-) | <-- Second Shell (n=2)
| |
-------
-------
| 13p+,14n0 | <-- Nucleus
-------
Remember, this is a simplified representation. The actual electron arrangement is far more complex, but the Bohr model serves as a good introductory visual.
Limitations of the Bohr Model
It's crucial to acknowledge the Bohr model's limitations:
-
Electron Orbits: The model depicts electrons orbiting the nucleus in fixed, circular paths. In reality, electrons exist in orbitals, regions of space with a high probability of finding an electron, and their movement is not confined to specific paths.
-
Energy Levels: While the model correctly predicts energy levels, it doesn't accurately represent the energy transitions between these levels. More sophisticated models, like the quantum mechanical model, are needed for precise predictions of electron behavior.
-
Electron-Electron Interactions: The Bohr model largely ignores the interactions between electrons within the same shell. These interactions play a crucial role in determining the overall electronic structure of the atom.
-
Multi-Electron Atoms: The model becomes progressively less accurate for atoms with many electrons. The complexity of electron-electron interactions makes it difficult for the simple Bohr model to adequately capture the behavior of electrons in these atoms.
Beyond the Bohr Model: A Glimpse into Quantum Mechanics
The quantum mechanical model provides a more accurate and comprehensive representation of atomic structure. Key concepts include:
-
Orbitals: Electrons occupy atomic orbitals, which are regions of space with a high probability of finding an electron. These orbitals have specific shapes and energy levels. For example, the 's' orbitals are spherical, while the 'p' orbitals are dumbbell-shaped.
-
Quantum Numbers: The quantum mechanical model uses four quantum numbers (principal, azimuthal, magnetic, and spin quantum numbers) to describe the state of an electron. These quantum numbers dictate an electron's energy level, orbital shape, orientation, and spin.
-
Electron Configuration: This describes how electrons are distributed among the different orbitals in an atom. For aluminum, the electron configuration is 1s²2s²2p⁶3s²3p¹. This indicates the filling of the orbitals according to specific rules (Aufbau principle, Hund's rule, Pauli exclusion principle).
-
Wave-Particle Duality: The quantum mechanical model acknowledges the dual nature of electrons, exhibiting both wave-like and particle-like properties.
Conclusion: The Bohr Model – A Stepping Stone to Understanding
The Bohr model, despite its limitations, serves as an excellent introductory tool for visualizing atomic structure. It provides a simplified representation of electron arrangement, making it easier to grasp fundamental concepts like electron shells and energy levels. Understanding the Bohr model is crucial before moving on to the more complex and accurate quantum mechanical model. By understanding its limitations, we can appreciate the advancements made in atomic theory and the sophistication of modern quantum mechanics. While the Bohr model for aluminum offers a basic understanding, remember to embrace the more comprehensive quantum mechanical model for a more accurate depiction of the atom's structure. This progression is key to a deeper understanding of chemistry and physics.
Latest Posts
Latest Posts
-
All Squares Are Rectangles But Not All Rectangles Are Squares
Apr 02, 2025
-
A Process By Which Information Is Exchanged Between Individuals
Apr 02, 2025
-
Select The Four Statements About Plasmodium That Are True
Apr 02, 2025
-
Greatest Common Factor Of 36 And 20
Apr 02, 2025
-
What Is The Antonym Of Urban
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Draw The Bohr Model For Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
