What Typ Of Ions Do Acids Release
News Leon
Mar 24, 2025 · 6 min read
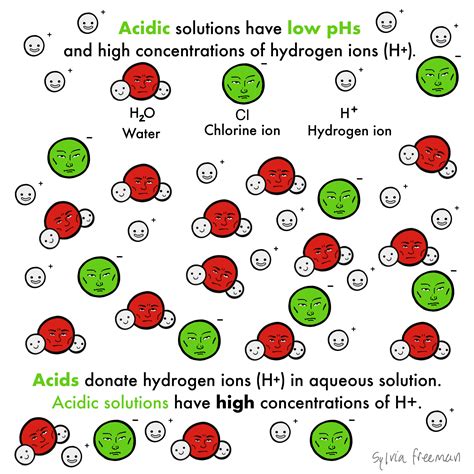
Table of Contents
What Types of Ions Do Acids Release? A Deep Dive into Acid-Base Chemistry
Acids are fundamental chemical compounds with a profound impact on various aspects of our lives, from the digestion of food to industrial processes. Understanding their properties, particularly their behavior in solution, is crucial in many scientific disciplines. This comprehensive article delves into the core characteristic of acids: the types of ions they release when dissolved in water. We will explore the different classifications of acids, the underlying mechanisms of ion release, and the implications of this behavior.
Understanding Acids and Ionization
Acids are substances that, when dissolved in water, increase the concentration of hydrogen ions (H⁺). This definition, while seemingly simple, encompasses a broad range of chemical species with diverse properties and applications. The crucial aspect here is the release of ions, specifically hydrogen ions, upon dissolution. This process is known as ionization or dissociation. It’s important to distinguish between ionization and dissociation: dissociation refers to the separation of already existing ions in a compound, while ionization involves the formation of ions from a neutral molecule. Many acids undergo ionization in water.
The Arrhenius Definition: A Foundation
The Arrhenius definition, a cornerstone of acid-base chemistry, defines an acid as a substance that produces hydrogen ions (H⁺) when dissolved in water. This definition, while historically significant, has limitations. It focuses solely on aqueous solutions and doesn't encompass acid-base reactions in non-aqueous solvents. However, it provides a valuable starting point for understanding the ion release behavior of acids.
The Brønsted-Lowry Definition: A Broader Perspective
The Brønsted-Lowry definition offers a more comprehensive understanding of acids. It defines an acid as a proton (H⁺) donor. This broader definition extends beyond aqueous solutions and encompasses reactions where a proton is transferred from one molecule to another. This definition acknowledges that the hydrogen ion, in reality, doesn't exist freely in solution but rather forms a hydronium ion (H₃O⁺) by interacting with water molecules. The equation below illustrates this:
HCl + H₂O → H₃O⁺ + Cl⁻
In this reaction, hydrochloric acid (HCl) acts as a Brønsted-Lowry acid by donating a proton to a water molecule, forming a hydronium ion and a chloride ion (Cl⁻). The key takeaway here is the release of a proton (and the concomitant formation of a conjugate base, Cl⁻ in this case).
Types of Acids and Their Ion Release Behavior
Acids are classified into various categories based on their properties and structure. Understanding these classifications helps predict the type and quantity of ions released upon ionization.
Strong Acids: Complete Ionization
Strong acids are substances that completely ionize in water, meaning that virtually all of the acid molecules dissociate into ions. This results in a high concentration of hydrogen ions (or hydronium ions) in the solution. Examples of strong acids include:
- Hydrochloric acid (HCl): Ionizes completely to form H⁺ (or H₃O⁺) and Cl⁻.
- Sulfuric acid (H₂SO₄): Ionizes in two steps. The first step is essentially complete, producing H⁺ (or H₃O⁺) and HSO₄⁻. The second step is less complete.
- Nitric acid (HNO₃): Ionizes completely to form H⁺ (or H₃O⁺) and NO₃⁻.
- Hydrobromic acid (HBr): Ionizes completely to form H⁺ (or H₃O⁺) and Br⁻.
- Perchloric acid (HClO₄): Ionizes completely to form H⁺ (or H₃O⁺) and ClO₄⁻.
The complete ionization of strong acids makes them highly effective in reactions requiring a high concentration of H⁺ ions.
Weak Acids: Partial Ionization
Weak acids only partially ionize in water, meaning that only a small fraction of the acid molecules dissociate into ions. This results in a lower concentration of hydrogen ions (or hydronium ions) compared to strong acids. The extent of ionization is often expressed as an acid dissociation constant (Ka). A lower Ka value indicates a weaker acid. Examples of weak acids include:
- Acetic acid (CH₃COOH): Ionizes to a small extent, producing CH₃COO⁻ and H⁺ (or H₃O⁺).
- Formic acid (HCOOH): Similar to acetic acid, it partially ionizes, releasing formate ion and H⁺ (or H₃O⁺).
- Hydrofluoric acid (HF): A relatively weak acid that ionizes to produce F⁻ and H⁺ (or H₃O⁺).
- Carbonic acid (H₂CO₃): A diprotic weak acid that ionizes in two steps, releasing bicarbonate (HCO₃⁻) and carbonate (CO₃²⁻) ions, along with H⁺ (or H₃O⁺).
- Phosphoric acid (H₃PO₄): A triprotic weak acid with three ionization steps, producing different phosphate ions and H⁺ (or H₃O⁺).
The incomplete ionization of weak acids makes them less effective in reactions requiring a high concentration of H⁺ ions. The equilibrium between the ionized and unionized forms plays a crucial role in their behavior.
Polyprotic Acids: Multiple Ionizations
Polyprotic acids are acids that can donate more than one proton (H⁺) per molecule. They undergo multiple ionization steps, releasing different anions at each step. The strength of each ionization step can vary. Examples include:
- Sulfuric acid (H₂SO₄): A diprotic acid.
- Phosphoric acid (H₃PO₄): A triprotic acid.
- Citric acid (C₆H₈O₇): A triprotic acid.
The stepwise ionization of polyprotic acids leads to a complex equilibrium system with multiple ionic species in solution.
Organic Acids: Carbon-Containing Acids
Many organic acids contain a carboxyl group (-COOH) which is responsible for their acidic properties. These acids can be either strong or weak, depending on their structure and the substituents attached to the carboxyl group. Examples include:
- Acetic acid (CH₃COOH): A weak monoprotic acid.
- Citric acid (C₆H₈O₇): A weak triprotic acid.
- Lactic acid (C₃H₆O₃): A weak monoprotic acid.
- Benzoic acid (C₇H₆O₂): A weak monoprotic acid.
The presence of electron-withdrawing groups near the carboxyl group generally increases the acidity, while electron-donating groups decrease it.
Implications of Ion Release
The release of ions by acids has profound implications in various fields:
- Chemistry: Understanding the ionization behavior of acids is crucial for predicting reaction outcomes, calculating equilibrium constants, and designing chemical processes.
- Biology: Acids play a vital role in biological systems, with many biological processes relying on the controlled release of H⁺ ions. For instance, the stomach uses hydrochloric acid for digestion.
- Environmental Science: Acid rain, caused by the release of acidic pollutants into the atmosphere, poses a significant threat to ecosystems.
- Industry: Acids are widely used in industrial processes, such as the production of fertilizers, plastics, and pharmaceuticals.
Factors Affecting Ion Release
Several factors influence the extent of ion release by acids:
- Acid Strength: Strong acids ionize completely, while weak acids ionize partially.
- Concentration: A higher concentration of acid generally leads to a higher concentration of H⁺ ions, though the percentage ionization may remain the same for a weak acid.
- Temperature: Increased temperature usually increases the degree of ionization for weak acids.
- Solvent: The nature of the solvent significantly influences the ionization of acids. Water is a common solvent, but other solvents can affect the extent of ionization.
Conclusion
The release of ions, primarily hydrogen ions (or hydronium ions), is a defining characteristic of acids. Understanding the types of ions released, the extent of ionization, and the factors influencing this process is fundamental to comprehending the behavior of acids in various contexts. From the strong acids that completely dissociate to the weak acids that only partially ionize, each type exhibits unique properties and plays a crucial role in chemical and biological systems. The concepts discussed here provide a solid foundation for further exploration into the fascinating world of acid-base chemistry. By understanding the nuances of ion release, we can better predict and control chemical reactions, design efficient processes, and appreciate the crucial role acids play in our world.
Latest Posts
Latest Posts
-
What Does Rstrip Do In Python
Mar 25, 2025
-
Figure A Shows An Element Of Length Ds
Mar 25, 2025
-
Is Stainless Steel A Homogeneous Mixture
Mar 25, 2025
-
Place The Following Parts Of A Reflex Arc In Order
Mar 25, 2025
-
How Many Valence Electrons In Cl
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Typ Of Ions Do Acids Release . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
