What Is The Solvent For Salt Water
News Leon
Apr 06, 2025 · 6 min read
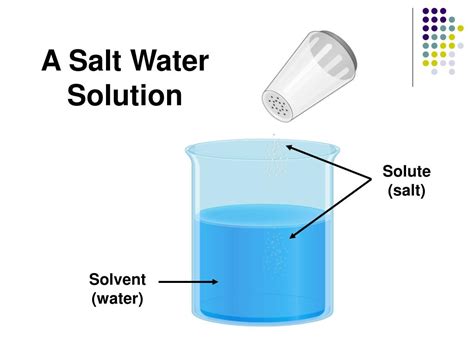
Table of Contents
What is the Solvent for Salt Water? Understanding Solutions and Solvation
Saltwater, a ubiquitous substance found in oceans, seas, and even some lakes, isn't just a simple mixture. It's a solution, a homogenous mixture composed of a solute dissolved in a solvent. Understanding this fundamental concept is key to grasping the nature of saltwater and many other solutions. This article will delve deep into the question: what is the solvent for saltwater, exploring the properties of solvents and solutes, the process of solvation, and the impact of various factors on the solubility of salt in water.
Defining Solvents and Solutes: The Foundation of Solutions
Before tackling the specific case of saltwater, let's clarify the terminology. A solution is a homogenous mixture where one substance, the solute, is uniformly dispersed within another substance, the solvent. The solvent is typically the component present in the larger amount. Imagine brewing tea: the tea leaves release their flavor compounds (the solute) into the hot water (the solvent), creating a homogenous tea solution.
The crucial characteristic of a solution is its homogeneity. This means that at a macroscopic level, the solute is evenly distributed throughout the solvent. You can't visually distinguish the solute from the solvent; a drop from anywhere in the cup of tea will taste the same. This differs from a suspension, where the solute particles are larger and may settle over time.
Water: The Universal Solvent
In the case of saltwater, the answer is clear: water is the solvent. Water's unique properties make it an exceptionally effective solvent for a vast array of substances, earning it the title of "universal solvent." This remarkable capability stems from its molecular structure and polarity.
The Polar Nature of Water: A Key to Solvation
Water (H₂O) is a polar molecule, meaning it has a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). This polarity arises from the unequal sharing of electrons between oxygen and hydrogen atoms, creating a dipole moment. This inherent polarity is the driving force behind water's ability to dissolve many ionic compounds, like salt.
Solvation: The Process of Dissolution
When salt (sodium chloride, NaCl) is added to water, the polar water molecules interact with the ions in the salt crystal. The slightly negative oxygen ends of water molecules attract the positively charged sodium ions (Na⁺), while the slightly positive hydrogen ends attract the negatively charged chloride ions (Cl⁻). This process is known as solvation, where solvent molecules surround and stabilize the solute ions, weakening the electrostatic forces holding the crystal lattice together.
The water molecules effectively shield the ions from each other, preventing them from recombining to form the solid crystal. The solvated ions are then free to move independently within the solution, resulting in a homogenous saltwater solution. This is why salt dissolves readily in water, creating a transparent solution.
Factors Affecting Salt Solubility in Water: A Deeper Dive
While water is an excellent solvent for salt, several factors can influence the extent to which salt dissolves in water:
Temperature: The Heat Factor
Generally, the solubility of most solids in liquids, including salt in water, increases with temperature. Higher temperatures provide the water molecules with greater kinetic energy, increasing their ability to overcome the attractive forces between the salt ions and effectively break apart the salt crystal. This leads to a higher concentration of dissolved salt at higher temperatures.
Pressure: A Minor Player
Pressure has a relatively minor effect on the solubility of solids in liquids like salt in water. While changes in pressure can significantly affect the solubility of gases in liquids, the impact on solid solubility is typically negligible under normal conditions.
Concentration: Saturation Point
Even though water is a good solvent for salt, there's a limit to how much salt can dissolve in a given amount of water at a specific temperature. This limit is called the saturation point. Once the saturation point is reached, adding more salt will not result in further dissolution; the excess salt will remain undissolved as a precipitate at the bottom of the container. A saturated solution is one where the maximum amount of solute has dissolved in the solvent at a given temperature and pressure.
Beyond Saltwater: Other Solutions and Solvents
The principles of solvation and solubility apply to a vast array of solutions beyond saltwater. Many other substances can act as solvents, dissolving various solutes depending on their properties.
Organic Solvents: A Diverse Group
Organic solvents, derived from carbon-containing compounds, represent a broad class of solvents used in various industrial and laboratory applications. Examples include ethanol, acetone, and hexane. Their solubility properties differ greatly from water; they often dissolve nonpolar substances effectively, while water typically dissolves polar substances.
Polar vs. Nonpolar Solvents: The "Like Dissolves Like" Rule
A fundamental principle in solubility is the "like dissolves like" rule. This means that polar solvents tend to dissolve polar solutes, while nonpolar solvents tend to dissolve nonpolar solutes. This principle stems from the interactions between the molecules; similar polarities lead to stronger attractive forces, facilitating dissolution.
Applications of Understanding Saltwater Solutions
The understanding of saltwater solutions has vast practical implications across many scientific fields and everyday applications:
Oceanography: Studying the Oceans
Oceanography heavily relies on understanding saltwater properties. Scientists study salinity (the salt concentration in water) to understand ocean currents, marine life distribution, and the impact of climate change. Salinity variations affect water density, influencing ocean circulation patterns.
Desalination: Providing Fresh Water
Desalination technologies aim to remove salt from seawater to provide fresh drinking water in regions with water scarcity. Understanding the properties of saltwater solutions is essential for developing efficient and cost-effective desalination methods. Processes like reverse osmosis rely on the principles of solution chemistry to separate salt from water.
Food Science: Salt in Food Preservation
Salt has been used for centuries as a food preservative. Understanding its solubility and interaction with food components is crucial for food science and technology. Salt inhibits microbial growth by altering osmotic pressure within the food, preventing spoilage.
Medicine: Intravenous Solutions
Intravenous (IV) solutions often include salts dissolved in water to maintain electrolyte balance in patients. Precise control of salt concentrations in IV fluids is critical for patient health and safety.
Conclusion: The Significance of Solvents
The solvent for saltwater is water, a ubiquitous and remarkably versatile substance. Understanding the principles of solvation, solubility, and the properties of both solvents and solutes is vital across a wide range of scientific disciplines and practical applications. From the vast oceans to medical treatments, the interaction between salt and water is a fundamental process with far-reaching consequences. Further research continues to expand our knowledge of solution chemistry, leading to innovative applications and advancements in various fields. The simplicity of the question, "What is the solvent for saltwater?" belies the complexity and significance of the underlying principles involved.
Latest Posts
Latest Posts
-
12 5 Of What Number Is 24
Apr 07, 2025
-
Is Molar Mass The Same As Molecular Mass
Apr 07, 2025
-
170 Rounded To The Nearest Hundred
Apr 07, 2025
-
Balanced Equation For Acetic Acid And Sodium Bicarbonate
Apr 07, 2025
-
Which Of The Following Is Input Device
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Solvent For Salt Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
