Is Molar Mass The Same As Molecular Mass
News Leon
Apr 07, 2025 · 6 min read
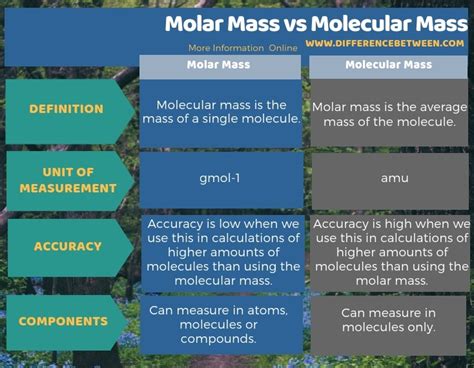
Table of Contents
Is Molar Mass the Same as Molecular Mass?
Understanding the concepts of molar mass and molecular mass is crucial for anyone working with chemistry, particularly in stoichiometry and quantitative analysis. While the terms are closely related and often used interchangeably in casual conversation, there's a subtle but significant difference. This article delves deep into the definitions, calculations, and applications of both molar mass and molecular mass, clarifying their relationship and highlighting their distinctions.
Defining Molecular Mass
Molecular mass, also known as molecular weight, refers to the mass of a single molecule of a substance. It's calculated by summing the atomic masses of all the atoms present in that molecule. Atomic mass, in turn, is the average mass of an atom of an element, taking into account the relative abundances of its isotopes. The unit for molecular mass is typically the atomic mass unit (amu) or Dalton (Da).
Example:
Let's consider water (H₂O). The atomic mass of hydrogen (H) is approximately 1 amu, and the atomic mass of oxygen (O) is approximately 16 amu. Therefore, the molecular mass of water is:
(2 × 1 amu) + 16 amu = 18 amu
Calculating Molecular Mass: A Step-by-Step Guide
-
Identify the chemical formula: Begin by determining the correct chemical formula of the molecule. For example, for glucose, the formula is C₆H₁₂O₆.
-
Find atomic masses: Consult a periodic table to find the atomic mass of each element present in the molecule. Remember these are average atomic masses, accounting for isotopic abundance.
-
Multiply and sum: Multiply the atomic mass of each element by the number of atoms of that element in the molecule. Then, add up all these products to obtain the total molecular mass.
Let's calculate the molecular mass of glucose (C₆H₁₂O₆):
- Carbon (C): Atomic mass ≈ 12 amu; 6 carbon atoms = 6 × 12 amu = 72 amu
- Hydrogen (H): Atomic mass ≈ 1 amu; 12 hydrogen atoms = 12 × 1 amu = 12 amu
- Oxygen (O): Atomic mass ≈ 16 amu; 6 oxygen atoms = 6 × 16 amu = 96 amu
Total molecular mass of glucose: 72 amu + 12 amu + 96 amu = 180 amu
Defining Molar Mass
Molar mass is defined as the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 × 10²³) of entities (atoms, molecules, ions, etc.). The unit for molar mass is typically grams per mole (g/mol).
The key difference lies here: molecular mass deals with the mass of a single molecule, while molar mass deals with the mass of a vast collection of molecules (a mole). The numerical value of molar mass is identical to the molecular mass, but the units are different. This is because a mole of a substance contains Avogadro's number of molecules. The molar mass provides a convenient way to relate the microscopic world (individual molecules) to the macroscopic world (measurable quantities in grams).
Example:
The molar mass of water (H₂O) is 18 g/mol. This means that 1 mole of water molecules weighs 18 grams.
Calculating Molar Mass: A Practical Approach
Calculating molar mass is essentially the same process as calculating molecular mass, except that the final result is expressed in grams per mole (g/mol) instead of atomic mass units (amu).
Let's reiterate the calculation for glucose (C₆H₁₂O₆):
Using the same steps as above, we find the total mass of one molecule of glucose to be 180 amu. Therefore, the molar mass of glucose is 180 g/mol.
The Relationship Between Molar Mass and Molecular Mass
The relationship between molar mass and molecular mass is direct and proportional: the numerical value of the molar mass is the same as the numerical value of the molecular mass, but expressed in different units. This crucial link allows for easy conversion between the mass of individual molecules and the mass of a macroscopic sample.
In essence, you can think of molar mass as a scaled-up version of molecular mass, converting from the microscopic scale (amu) to the macroscopic scale (grams). The scaling factor is Avogadro's number.
Applications of Molar Mass and Molecular Mass
Both molecular mass and molar mass are essential concepts in various areas of chemistry and related fields:
-
Stoichiometry: Molar mass is indispensable for performing stoichiometric calculations, allowing us to determine the amounts of reactants and products in chemical reactions. This is crucial for balancing chemical equations and calculating yields.
-
Quantitative analysis: Determining the molar mass of an unknown compound is a key aspect of identifying its chemical formula through various analytical techniques.
-
Solution preparation: Molar mass is used to prepare solutions of specific concentrations (e.g., molarity, molality).
-
Polymer chemistry: Determining the molar mass of polymers is crucial for understanding their properties and behavior.
-
Biochemistry: Molecular and molar masses are crucial in understanding the properties and interactions of biological macromolecules such as proteins and nucleic acids.
-
Pharmaceutical science: Determining the molar mass of drugs is fundamental for dosage calculations and formulation development.
-
Environmental science: Molar mass is used in determining the concentrations of pollutants and other substances in environmental samples.
Molar Mass vs. Molecular Mass in Different Chemical Species
The distinction between molar mass and molecular mass becomes even clearer when we consider different types of chemical species:
1. Covalent Compounds: Covalent compounds, such as water (H₂O) and glucose (C₆H₁₂O₆), exist as discrete molecules. Both molecular mass and molar mass are readily defined and easily calculated using the methods described above.
2. Ionic Compounds: Ionic compounds, such as sodium chloride (NaCl), do not exist as discrete molecules. Instead, they form a crystal lattice of ions. In this case, the term "molecular mass" is not strictly applicable. However, we can still define a molar mass based on the formula unit of the compound. The molar mass of NaCl is the sum of the molar masses of sodium (Na) and chlorine (Cl) ions, yielding approximately 58.44 g/mol.
3. Elements: For elemental substances, the molar mass is equal to the atomic mass expressed in grams per mole. For example, the molar mass of carbon (C) is 12 g/mol.
Common Misconceptions and Clarifications
One frequent misconception is that molar mass and molecular mass are interchangeable. While the numerical values are the same, the units and the underlying meaning differ significantly. Remember, molar mass relates to a macroscopic amount of substance (one mole), while molecular mass relates to a single molecule.
Another common confusion arises with the terminology used for ionic compounds. Since ionic compounds don't form discrete molecules, the term "formula mass" or "formula weight" is sometimes used instead of "molecular mass." However, the term "molar mass" remains appropriate and consistently applies to all types of chemical substances.
Conclusion
In conclusion, while the numerical values of molar mass and molecular mass are identical, they represent fundamentally different concepts. Molecular mass refers to the mass of a single molecule, measured in atomic mass units (amu). Molar mass refers to the mass of one mole of a substance, measured in grams per mole (g/mol). Understanding the distinction between these two concepts is critical for accurate and meaningful chemical calculations and interpretations across diverse applications in chemistry and related fields. Mastering these concepts is vital for anyone seeking a deep understanding of chemical principles and their practical applications.
Latest Posts
Latest Posts
-
What Type Of Rock Are Fossils Mostly Found In
Apr 10, 2025
-
2 Rays That Meet At An Endpoint
Apr 10, 2025
-
How Many Calvin Cycles To Make 1 Glucose
Apr 10, 2025
-
An Electron Has A Charge Of
Apr 10, 2025
-
How Does The Endoplasmic Reticulum Provide Mechanical Support
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Is Molar Mass The Same As Molecular Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.