Balanced Equation For Acetic Acid And Sodium Bicarbonate
News Leon
Apr 07, 2025 · 7 min read
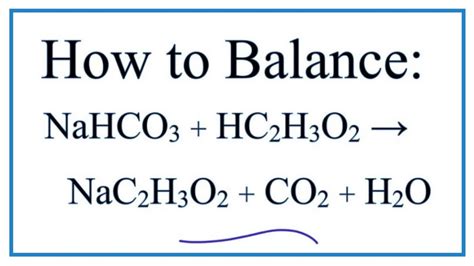
Table of Contents
The Balanced Equation for Acetic Acid and Sodium Bicarbonate: A Deep Dive
The reaction between acetic acid (CH₃COOH) and sodium bicarbonate (NaHCO₃) is a classic example of an acid-base neutralization reaction, frequently encountered in chemistry labs and everyday life. Understanding the balanced equation for this reaction, along with its implications and applications, is crucial for various scientific and practical purposes. This comprehensive article will delve into the intricacies of this reaction, exploring the balanced equation, its stoichiometry, the products formed, and its relevance in different contexts.
Understanding the Reactants: Acetic Acid and Sodium Bicarbonate
Before diving into the reaction itself, let's briefly review the properties of the reactants: acetic acid and sodium bicarbonate.
Acetic Acid (CH₃COOH)
Acetic acid, also known as ethanoic acid, is a weak organic acid with the chemical formula CH₃COOH. It's a colorless liquid with a pungent, vinegar-like odor. The "weak" designation signifies that it doesn't fully dissociate into ions in aqueous solution, unlike strong acids like hydrochloric acid (HCl). Acetic acid's acidic properties stem from the readily available proton (H⁺) on the carboxyl group (-COOH).
Sodium Bicarbonate (NaHCO₃)
Sodium bicarbonate, commonly known as baking soda, is a white crystalline powder with the chemical formula NaHCO₃. It's an amphoteric compound, meaning it can act as both an acid and a base, depending on the reaction conditions. In its reaction with acetic acid, it acts as a base, accepting a proton from the acid. This ability to neutralize acids is the basis for many of its applications.
The Balanced Chemical Equation
The reaction between acetic acid and sodium bicarbonate is a neutralization reaction, producing sodium acetate, water, and carbon dioxide. The balanced chemical equation is:
CH₃COOH(aq) + NaHCO₃(aq) → CH₃COONa(aq) + H₂O(l) + CO₂(g)
Let's break down the equation:
- CH₃COOH(aq): Acetic acid in aqueous solution (aq) indicates it's dissolved in water.
- NaHCO₃(aq): Sodium bicarbonate in aqueous solution.
- CH₃COONa(aq): Sodium acetate, a salt formed by the neutralization reaction, also in aqueous solution.
- H₂O(l): Water, in its liquid state (l).
- CO₂(g): Carbon dioxide gas, in its gaseous state (g).
The equation is balanced because the number of atoms of each element is equal on both the reactant and product sides. We have:
- 2 Carbon atoms on both sides
- 4 Hydrogen atoms on both sides
- 4 Oxygen atoms on both sides
- 1 Sodium atom on both sides
Stoichiometry of the Reaction
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. In the acetic acid and sodium bicarbonate reaction, the stoichiometric ratio is 1:1. This means that one mole of acetic acid reacts completely with one mole of sodium bicarbonate to produce one mole each of sodium acetate, water, and carbon dioxide.
This 1:1 ratio is crucial for understanding the reaction's quantitative aspects. For example, if we know the amount of acetic acid used, we can calculate the exact amount of sodium bicarbonate needed for complete neutralization, and vice-versa. This principle is fundamental in titrations and other quantitative chemical analyses.
The Products of the Reaction
The reaction yields three products:
Sodium Acetate (CH₃COONa)
Sodium acetate is a salt formed by the neutralization of acetic acid's acidic proton with the sodium ion from sodium bicarbonate. It's a soluble ionic compound, meaning it readily dissolves in water. Sodium acetate solutions are mildly alkaline due to the acetate ion's weak basic properties. It has numerous applications, including use as a buffer in chemical systems, a food preservative, and in the textile industry.
Water (H₂O)
Water is a byproduct of the neutralization reaction. The hydroxide ion (OH⁻) from the dissociation of sodium bicarbonate combines with the proton (H⁺) from acetic acid to form water. The formation of water is a key indicator of a successful acid-base neutralization reaction.
Carbon Dioxide (CO₂)
Carbon dioxide is a gas released during the reaction. The bicarbonate ion (HCO₃⁻) decomposes into carbonate (CO₃²⁻) and releases a proton (H⁺), which reacts with the hydroxide ion to form water. The resulting carbonate is unstable and decomposes into carbon dioxide gas, which is then released. This gas evolution is often observed as effervescence or bubbling when the reaction takes place. This visible gas production is a characteristic feature of this reaction and helps in confirming its completion.
Applications of the Reaction
The reaction between acetic acid and sodium bicarbonate has several practical applications:
Baking
In baking, sodium bicarbonate acts as a leavening agent. When mixed with acidic ingredients like buttermilk or vinegar (which contains acetic acid), the reaction produces carbon dioxide gas. This gas creates bubbles within the batter, causing it to rise and resulting in a lighter, fluffier texture in baked goods.
Antacid Relief
Sodium bicarbonate is a common ingredient in antacids. Its ability to neutralize stomach acid (which is primarily hydrochloric acid) helps to relieve heartburn and indigestion. Although the reaction with stomach acid is different from the one with acetic acid, the principle of acid-neutralization remains the same.
Chemical Buffers
The reaction can be used to create buffer solutions in chemical experiments and processes. Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base. A mixture of acetic acid and sodium acetate (a product of this reaction) can act as an effective buffer system.
Cleaning
The reaction's ability to produce carbon dioxide gas can be exploited for cleaning purposes. For instance, it's sometimes used to remove stubborn stains or to unclog drains. The bubbling action helps to loosen and lift away dirt and debris.
Scientific Experiments
The reaction serves as a valuable demonstration in chemistry education to illustrate concepts like acid-base neutralization, stoichiometry, gas evolution, and the properties of weak acids and bases. Students can perform experiments to quantify the amount of reactants and products and learn how to interpret observations like gas evolution and pH changes.
Factors Affecting the Reaction Rate
Several factors influence the rate at which the reaction between acetic acid and sodium bicarbonate proceeds:
Concentration of Reactants
Higher concentrations of both acetic acid and sodium bicarbonate lead to a faster reaction rate. A greater number of reactant molecules increase the frequency of collisions, leading to a more rapid reaction.
Temperature
Increasing the temperature generally accelerates the reaction rate. Higher temperatures provide the reactant molecules with more kinetic energy, increasing the likelihood of successful collisions and thus speeding up the reaction.
Surface Area
While less relevant in this specific liquid-liquid reaction, surface area plays a role in reactions involving solids. If one reactant were a solid, a larger surface area would expose more reactant molecules to interaction, accelerating the reaction.
Presence of Catalysts
Catalysts can also affect the reaction rate. A catalyst would lower the activation energy of the reaction, allowing it to proceed more quickly. However, it's unlikely a catalyst is needed for this reaction.
Safety Precautions
When conducting experiments involving acetic acid and sodium bicarbonate, it's crucial to observe safety precautions:
- Always wear appropriate safety goggles to protect your eyes.
- Handle chemicals carefully to prevent spills and skin contact.
- Conduct experiments in a well-ventilated area as carbon dioxide gas is produced.
- If spills occur, immediately clean up using appropriate procedures.
Conclusion
The reaction between acetic acid and sodium bicarbonate is a simple yet insightful example of an acid-base neutralization reaction with numerous practical applications. Understanding the balanced equation, stoichiometry, products, and influencing factors is crucial in various scientific and industrial contexts. The reaction's widespread use in baking, antacids, buffer solutions, and cleaning demonstrates its versatility and significance in our daily lives. By carefully observing safety precautions, this reaction can also serve as an excellent demonstration in chemistry education to enhance the understanding of fundamental chemical principles.
Latest Posts
Latest Posts
-
An Apple Falls From A Tree
Apr 10, 2025
-
What Type Of Rock Are Most Fossils Found In
Apr 10, 2025
-
What Is The Iupac Name Of This Alkane
Apr 10, 2025
-
Write The Electron Configuration For A Neutral Atom Of Bromine
Apr 10, 2025
-
An Air Filled Capacitor Consists Of Two Parallel
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Acetic Acid And Sodium Bicarbonate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.