What Is The Electron Configuration Of Ni
News Leon
Mar 25, 2025 · 5 min read
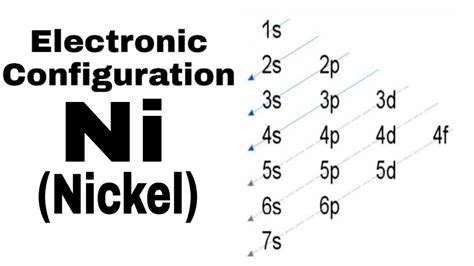
Table of Contents
What is the Electron Configuration of Ni? A Deep Dive into Nickel's Atomic Structure
Nickel (Ni), a silvery-white metal known for its strength, ductility, and corrosion resistance, holds a significant place in various industries. Understanding its electronic structure, particularly its electron configuration, is crucial to comprehending its chemical behavior and properties. This article provides a comprehensive exploration of nickel's electron configuration, delving into the underlying principles and its implications.
Understanding Electron Configuration
Before diving into nickel's specific electron configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. It follows the Aufbau principle, which dictates that electrons fill the lowest energy levels first. This principle, along with Hund's rule (electrons individually occupy each orbital within a subshell before doubling up) and the Pauli exclusion principle (no two electrons can have the same four quantum numbers), determines the arrangement. The electron configuration is typically represented using a notation that indicates the principal energy level (n), the subshell (s, p, d, or f), and the number of electrons in each subshell.
The Significance of Electron Configuration
Knowing the electron configuration is paramount for predicting an element's:
- Chemical reactivity: The valence electrons (electrons in the outermost shell) directly influence an element's ability to form chemical bonds.
- Physical properties: Properties like melting point, boiling point, and conductivity are closely tied to the electronic structure.
- Magnetic properties: The arrangement of electrons determines whether an element will exhibit paramagnetism (weak attraction to a magnetic field) or diamagnetism (weak repulsion from a magnetic field). Nickel is a prime example of an element with significant magnetic properties.
- Spectroscopic behavior: Electron transitions between energy levels are responsible for the absorption and emission of light, which is fundamental to spectroscopic techniques used for identifying and analyzing substances.
Determining the Electron Configuration of Nickel (Ni)
Nickel has an atomic number of 28, meaning a neutral nickel atom contains 28 protons and 28 electrons. To determine its electron configuration, we systematically fill the electron shells and subshells according to the Aufbau principle.
The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Therefore, the electron configuration of nickel is: 1s²2s²2p⁶3s²3p⁶4s²3d⁸.
Let's break this down:
- 1s²: The first energy level (n=1) contains one subshell (s), holding a maximum of two electrons. Nickel has two electrons in this subshell.
- 2s²: The second energy level (n=2) starts with the s subshell, again holding two electrons.
- 2p⁶: The second energy level also includes the p subshell, which can accommodate up to six electrons. Nickel fills this subshell completely.
- 3s²: The third energy level (n=3) begins with the s subshell, filled with two electrons.
- 3p⁶: The p subshell in the third energy level is also completely filled with six electrons.
- 4s²: The fourth energy level (n=4) starts with the s subshell, containing two electrons.
- 3d⁸: Finally, we reach the d subshell in the third energy level. A d subshell can hold up to ten electrons, and nickel has eight electrons in this subshell.
This complete configuration, 1s²2s²2p⁶3s²3p⁶4s²3d⁸, represents the ground state electron configuration of nickel.
Exceptions and Subtleties in Electron Configuration
While the Aufbau principle provides a general guideline, some elements exhibit exceptions. This is often due to the relatively small energy difference between certain subshells. While not strictly an exception in the traditional sense, it's important to note the subtle nuances of filling the 3d and 4s orbitals.
In many transition metals, including nickel, the energy difference between the 4s and 3d orbitals is small. This means that in some cases, it's energetically favorable for electrons to occupy the 3d orbital before completely filling the 4s orbital, or for an electron to be promoted from the 4s to 3d orbital in certain ions. This aspect is pivotal in understanding nickel's chemical and magnetic properties.
Nickel's Ions and their Electron Configurations
When nickel loses electrons to form ions (cations), it generally loses the 4s electrons first, before losing the 3d electrons. For example:
- Ni²⁺: The electron configuration becomes [Ar] 3d⁸. It has lost two electrons from the 4s orbital.
- Ni³⁺: The electron configuration is generally considered [Ar] 3d⁷, although subtle variations can occur depending on the ligand field.
These changes in electron configuration when forming ions directly impact the bonding and reactivity of nickel compounds.
The Role of Electron Configuration in Nickel's Properties
The specific electron configuration of nickel is responsible for many of its characteristic properties:
Magnetic Properties: Paramagnetism
Nickel's partially filled 3d subshell (with eight electrons) is the reason behind its paramagnetic nature. Unpaired electrons in the 3d orbitals create a net magnetic moment, leading to an attraction to an external magnetic field. This property is extensively utilized in various applications, from magnets to magnetic recording media.
Catalytic Activity
The availability of d orbitals in nickel facilitates its catalytic activity. The ability of nickel to accommodate electrons during chemical reactions makes it an efficient catalyst in various processes, including hydrogenation, carbonylation, and reforming reactions.
Alloying Behavior
Nickel's ability to form alloys with other metals stems from its electron configuration. Its electron structure allows it to readily interact with other metals, resulting in alloys exhibiting enhanced properties like strength, corrosion resistance, and ductility. This is pivotal in numerous industries, including aerospace and automotive.
Metallic Bonding
The metallic bond, responsible for many of nickel's metallic characteristics (high conductivity, malleability, ductility), is a direct consequence of the arrangement of its electrons. The delocalized valence electrons contribute to the strong cohesive forces within the metallic structure.
Conclusion: Understanding Nickel Through its Electron Configuration
The electron configuration of nickel, 1s²2s²2p⁶3s²3p⁶4s²3d⁸, is far more than just a symbolic representation. It's a key to understanding its properties and behavior. From its magnetic properties to its catalytic activity and alloying capabilities, the arrangement of electrons in nickel directly impacts its applications in various fields. This detailed analysis demonstrates how understanding electron configuration is fundamental to comprehending the characteristics and applications of elements, solidifying the essential role of atomic structure in chemistry and materials science. Further research and exploration into the subtleties of electron configuration in transition metals like nickel continue to drive advancements in numerous scientific and technological areas.
Latest Posts
Latest Posts
-
Ecg Is A Graphic Recording Of
Mar 26, 2025
-
The Most Abundant Type Of Immunoglobulin Is
Mar 26, 2025
-
What Does The Slope Of A Distance Time Graph Represent
Mar 26, 2025
-
What Is The Order Of Rotational Symmetry Of A Rhombus
Mar 26, 2025
-
A Wire With A Resistance Of 6 0 Ohm Is Drawn
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Ni . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
