What Is The Electron Configuration Of Li+
News Leon
Mar 30, 2025 · 6 min read
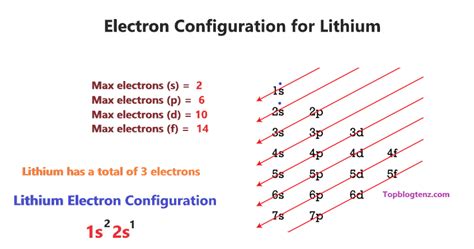
Table of Contents
What is the Electron Configuration of Li+?
Lithium, with its atomic number of 3, holds a unique position in the periodic table as the first element in the alkali metal group. Understanding its electron configuration, especially that of its cation Li+, is crucial for comprehending its chemical behavior and properties. This article delves deep into the electron configuration of Li+, exploring its derivation, significance, and implications within the context of atomic structure and chemical bonding.
Understanding Atomic Structure and Electron Configuration
Before we dive into the specifics of Li+, let's establish a foundational understanding of atomic structure and electron configuration. Atoms are composed of a nucleus containing protons and neutrons, surrounded by orbiting electrons. The number of protons defines the element's atomic number, and in a neutral atom, this number is equal to the number of electrons.
Electron configuration describes the arrangement of electrons within an atom's electron shells and subshells. These shells and subshells are regions of space around the nucleus where electrons are most likely to be found. They are designated by principal quantum numbers (n = 1, 2, 3, etc.), which represent energy levels, and azimuthal quantum numbers (l = 0, 1, 2, …, n-1), which represent subshells (s, p, d, f). Each subshell can hold a specific number of electrons: s subshells hold 2 electrons, p subshells hold 6, d subshells hold 10, and f subshells hold 14.
The Aufbau principle dictates that electrons fill orbitals in order of increasing energy. The order generally follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on. However, exceptions exist due to variations in orbital energies. Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. Finally, the Pauli exclusion principle asserts that no two electrons in an atom can have the same set of four quantum numbers.
Electron Configuration of Neutral Lithium (Li)
Neutral lithium (Li) has an atomic number of 3, meaning it possesses three protons and, in its neutral state, three electrons. Following the Aufbau principle, the electron configuration of neutral lithium is 1s²2s¹. This indicates that two electrons occupy the 1s orbital (the lowest energy level), and one electron occupies the 2s orbital. This outer electron in the 2s orbital is responsible for lithium's reactivity.
Ionization and the Formation of Li+
Lithium readily loses its single valence electron to achieve a stable, noble gas configuration resembling helium (1s²). This process is known as ionization, and it results in the formation of a positively charged ion, Li+. The loss of this electron involves overcoming the electrostatic attraction between the electron and the nucleus. The energy required for this process is called the ionization energy.
Electron Configuration of Li+
Since Li+ has lost one electron, it now has only two electrons. These two electrons fill the 1s orbital completely. Therefore, the electron configuration of Li+ is 1s². This configuration is isoelectronic with helium, meaning it has the same number of electrons as helium. This is a highly stable electron configuration, explaining the stability and relative inertness of the Li+ ion.
Significance of the Electron Configuration of Li+
The 1s² electron configuration of Li+ has several significant implications:
-
Chemical Stability: The full 1s subshell provides significant chemical stability to Li+. This means Li+ is less reactive than neutral lithium and is less likely to participate in further electron transfer reactions.
-
Ionic Bonding: Li+ frequently participates in ionic bonding, where it forms electrostatic attractions with negatively charged ions (anions). The stable electron configuration of Li+ makes it a strong participant in ionic compounds, contributing to the crystal structure and properties of these compounds.
-
Spectroscopy: The electron configuration influences the spectroscopic properties of Li+. The energy difference between electronic energy levels determines the wavelengths of light that Li+ can absorb or emit, allowing for spectroscopic identification.
-
Predicting Chemical Behavior: Knowing the electron configuration helps predict the chemical behavior of Li+ and its compounds. The stable octet configuration makes its reactions predictable, particularly in formation of ionic compounds with halides, oxides and other anions.
-
Understanding Reactivity: The difference between the electron configuration of Li and Li+ highlights the driving force behind the chemical reactivity of lithium. The drive to achieve a stable noble gas configuration is a fundamental principle in chemical bonding.
Comparing Li and Li+
The following table summarizes the key differences between neutral lithium and the lithium cation:
| Feature | Li (Neutral Lithium) | Li+ (Lithium Cation) |
|---|---|---|
| Atomic Number | 3 | 3 |
| Number of Protons | 3 | 3 |
| Number of Electrons | 3 | 2 |
| Electron Configuration | 1s²2s¹ | 1s² |
| Charge | 0 (Neutral) | +1 (Positive) |
| Reactivity | Highly reactive | Relatively unreactive |
| Chemical Bonding | Primarily forms ionic bonds | Primarily forms ionic bonds |
Applications of Li+
Lithium ions play a crucial role in various applications, driven by their unique properties stemming directly from their electron configuration:
-
Lithium-ion batteries: Li+ ions are essential components of lithium-ion batteries, which are ubiquitous in portable electronic devices, electric vehicles, and grid-scale energy storage. The movement of Li+ ions between the anode and cathode during charge and discharge processes is central to the battery's functionality.
-
Medicine: Lithium salts are used in the treatment of bipolar disorder. The precise mechanism of action is not fully understood, but it is thought to involve interactions with cellular processes and neurotransmission.
-
Ceramics and Glass: Li+ ions can be incorporated into ceramic and glass materials to modify their properties, such as thermal expansion coefficient and conductivity.
-
Lubricants: Li+ salts are used as additives in lubricating greases. They provide improved stability, high-temperature performance, and water resistance.
Conclusion
The electron configuration of Li+, 1s², is a direct consequence of lithium's ionization. This simple yet profound configuration underscores the principle of achieving noble gas configuration for chemical stability. The stability of Li+ significantly impacts its chemical behavior, making it a cornerstone in various applications, from high-tech lithium-ion batteries to essential medical treatments. Understanding this electron configuration provides a critical foundation for comprehending the chemistry of lithium and its widespread applications in modern technology and beyond. Further research continues to unveil new and exciting applications for this highly versatile element. The simplicity of its cation's electronic structure belies its complex and vital roles in a variety of fields, highlighting the power of fundamental atomic principles in shaping technological advancements and scientific discoveries.
Latest Posts
Latest Posts
-
Which Enzyme Is Not Involved In Dna Replication
Apr 01, 2025
-
A Person Makes A Quantity Of Iced Tea By Mixing
Apr 01, 2025
-
State The Law Of Conservation Of Energy Class 9
Apr 01, 2025
-
Number Of Protons Neutrons And Electrons In Beryllium
Apr 01, 2025
-
What Is The Bond Order Of Li2
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Li+ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
