State The Law Of Conservation Of Energy Class 9
News Leon
Apr 01, 2025 · 6 min read
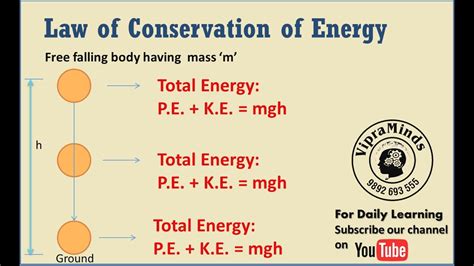
Table of Contents
The Law of Conservation of Energy: A Class 9 Exploration
The Law of Conservation of Energy is a fundamental principle in physics, stating that energy cannot be created or destroyed, only transformed from one form to another. This principle underpins our understanding of how the universe works, from the smallest atoms to the largest galaxies. Understanding this law is crucial for anyone pursuing a deeper understanding of science, particularly at the Class 9 level and beyond. This comprehensive guide will explore this vital concept, breaking it down into digestible parts and providing examples to illustrate its importance.
What is Energy?
Before diving into the law itself, let's clarify what we mean by "energy." Energy is the capacity to do work. Work, in a physics context, refers to the application of a force over a distance. This capacity manifests in various forms, each with its own characteristics:
Forms of Energy:
-
Kinetic Energy: The energy an object possesses due to its motion. A speeding car, a flying bird, even the atoms vibrating within a solid all possess kinetic energy. The faster the object moves, the greater its kinetic energy.
-
Potential Energy: Stored energy that has the potential to be converted into other forms of energy. This comes in several types:
- Gravitational Potential Energy: Energy stored due to an object's position in a gravitational field. An object held high above the ground possesses gravitational potential energy that's converted into kinetic energy as it falls.
- Elastic Potential Energy: Energy stored in a stretched or compressed object, like a stretched rubber band or a compressed spring.
- Chemical Potential Energy: Energy stored within the chemical bonds of molecules. This is released during chemical reactions, such as burning fuel or digesting food.
-
Thermal Energy (Heat): The total kinetic energy of the particles within a substance. Higher temperatures mean faster particle movement and thus higher thermal energy.
-
Light Energy (Radiant Energy): Energy carried by electromagnetic waves, including visible light, ultraviolet radiation, and infrared radiation.
-
Sound Energy: Energy transmitted through vibrations in a medium, such as air or water.
-
Electrical Energy: Energy associated with the flow of electric charge.
-
Nuclear Energy: Energy stored within the nucleus of an atom, released during nuclear reactions like fission or fusion.
The Law of Conservation of Energy Explained
The Law of Conservation of Energy states that the total energy of an isolated system remains constant. This means that energy cannot be created or destroyed; it simply changes form. In any energy transformation, the total amount of energy before the transformation equals the total amount of energy after the transformation. This is a crucial concept to grasp, as it governs all physical processes.
Key Implications:
-
Energy Transformation: Energy constantly changes form. For example, when you ride a bicycle, the chemical energy stored in your muscles is converted into kinetic energy (the motion of the bicycle) and thermal energy (heat produced by friction).
-
No Energy Loss: While energy changes form, the total amount remains constant. This doesn't mean there's no apparent loss. Some energy might be transformed into forms that are difficult to measure or utilize, such as heat dissipated into the environment. However, the total energy remains the same.
-
Isolated System: The law applies to isolated systems – systems that don't exchange energy with their surroundings. In reality, perfectly isolated systems are rare. However, the concept of an isolated system helps simplify the analysis of energy transformations.
Examples of the Law of Conservation of Energy
Let's illustrate the law with some everyday examples:
1. Rolling Ball:
Imagine a ball rolling down a hill. Initially, it possesses gravitational potential energy due to its height. As it rolls down, this potential energy is converted into kinetic energy, increasing its speed. At the bottom of the hill, it has maximum kinetic energy and minimum potential energy. Ignoring friction, the total energy (potential + kinetic) remains constant throughout the process.
2. Burning a Candle:
When a candle burns, the chemical potential energy stored in the wax is converted into light energy, thermal energy (heat), and a small amount of sound energy. While the forms of energy change, the total energy remains constant. The heat and light are released into the surroundings, but the total energy in the system (candle + surroundings) is conserved.
3. Hydroelectric Power Plant:
In a hydroelectric power plant, the gravitational potential energy of water stored behind a dam is converted into kinetic energy as the water flows downhill through turbines. The turbines, in turn, drive generators that convert this kinetic energy into electrical energy. Again, the total energy remains constant, with potential energy transforming into kinetic energy and then electrical energy.
4. Photosynthesis:
Plants utilize light energy from the sun to convert carbon dioxide and water into glucose (a sugar) and oxygen. This process converts light energy into chemical potential energy stored within the glucose molecules. The total energy remains constant.
5. Simple Pendulum:
A simple pendulum demonstrates the continuous conversion between potential and kinetic energy. At its highest point, the pendulum bob has maximum potential energy and minimum kinetic energy. As it swings down, potential energy is converted to kinetic energy, reaching maximum kinetic energy at the bottom of its swing. The process reverses as it swings back up. Ignoring air resistance, the total energy remains constant.
Applications of the Law of Conservation of Energy
The Law of Conservation of Energy has numerous applications in various fields:
-
Engineering: Design of machines and power systems relies heavily on understanding how energy is converted and transferred. Engineers use this principle to optimize efficiency and minimize energy waste.
-
Environmental Science: Understanding energy transformations helps us analyze environmental processes, such as climate change and energy resource management.
-
Medicine: Metabolic processes in the human body involve intricate energy transformations. Medical professionals utilize this knowledge to diagnose and treat metabolic disorders.
-
Astronomy: The study of stars and galaxies relies on understanding energy production and release through nuclear fusion.
Exceptions and Limitations
While the Law of Conservation of Energy is a fundamental principle, it's essential to acknowledge some nuances:
-
Relativity: Einstein's theory of relativity shows that mass and energy are equivalent (E=mc²). This implies that a small amount of mass can be converted into a large amount of energy, and vice versa. Nuclear reactions are a prime example of this mass-energy equivalence. While seemingly violating the strict interpretation of conservation, the combined mass-energy is conserved.
-
Non-isolated Systems: The law applies strictly to isolated systems. In real-world scenarios, systems rarely remain completely isolated, leading to energy exchange with the surroundings. However, for many practical purposes, we can approximate systems as isolated and still achieve accurate results.
Conclusion
The Law of Conservation of Energy is a cornerstone of physics, providing a fundamental framework for understanding energy transformations in various systems. From simple mechanical systems to complex biological processes, this law governs the flow of energy within our universe. While nuances exist, particularly concerning mass-energy equivalence, the principle of conservation remains central to our scientific understanding of the world around us. A thorough grasp of this law is essential for students at the Class 9 level and forms the bedrock for further study in physics and related disciplines. By understanding the various forms of energy and how they interconvert, we can better appreciate the intricate workings of the universe and the importance of energy efficiency in our daily lives. The examples provided serve as a springboard for further exploration and deeper understanding of this critical scientific principle.
Latest Posts
Latest Posts
-
Domain And Range Y 1 X
Apr 02, 2025
-
What Is The Best Topic For Speech
Apr 02, 2025
-
Why Is The Vacuole Larger In Plant Cells
Apr 02, 2025
-
How To Initialize A Tuple In Python
Apr 02, 2025
-
Find The Acceleration When The Velocity Is 0
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about State The Law Of Conservation Of Energy Class 9 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
