Number Of Protons Neutrons And Electrons In Beryllium
News Leon
Apr 01, 2025 · 5 min read
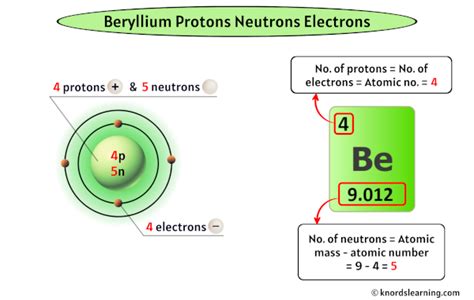
Table of Contents
Delving Deep into Beryllium: Protons, Neutrons, and Electrons
Beryllium, a fascinating element with a unique set of properties, occupies a significant place in the periodic table. Understanding its atomic structure, particularly the number of protons, neutrons, and electrons, is crucial to grasping its behavior and applications. This comprehensive guide will explore beryllium's atomic composition in detail, examining its isotopes, their stability, and the implications of its electron configuration for its chemical reactivity.
Understanding Atomic Structure: The Basics
Before we delve into the specifics of beryllium, let's review the fundamental components of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element's atomic number and dictates its position on the periodic table.
- Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. The number of neutrons, combined with the number of protons, determines the atom's mass number (isotopes).
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom. Electron configuration dictates an element's chemical behavior.
Beryllium's Atomic Structure: The Key Numbers
Beryllium (Be), with its atomic number of 4, always possesses four protons in its nucleus. This is non-negotiable; it's what makes it beryllium. A neutral beryllium atom also contains four electrons, balancing the positive charge of the protons. These electrons are arranged in two energy levels: two in the first shell (closest to the nucleus) and two in the second shell. This full inner shell contributes to beryllium's relatively high ionization energy.
The number of neutrons in beryllium, however, is variable. This variation leads to the existence of different isotopes.
Isotopes of Beryllium: Variations on a Theme
Isotopes are atoms of the same element (same number of protons) but with differing numbers of neutrons. This results in variations in their mass number (protons + neutrons). Beryllium has several isotopes, but the most important are:
-
Beryllium-9 (⁹Be): This is the most abundant and stable isotope of beryllium, comprising nearly 100% of naturally occurring beryllium. It has four protons and five neutrons. Its stability is due to a favorable neutron-to-proton ratio within its nucleus.
-
Beryllium-7 (⁷Be): A radioactive isotope with a relatively short half-life. It contains four protons and three neutrons. ⁷Be decays through electron capture or positron emission, transforming into Lithium-7. Its presence in certain astrophysical environments is noteworthy.
-
Beryllium-10 (¹⁰Be): Another radioactive isotope, 10Be is produced in the atmosphere by cosmic ray spallation. It has a much longer half-life than ⁷Be and is used in various dating techniques, particularly in studies of geological processes and climate change. It has four protons and six neutrons.
Other beryllium isotopes exist, but they are highly unstable and have extremely short half-lives. These are primarily observed in laboratory settings.
The Significance of Isotopic Abundance and Stability
The prevalence of ⁹Be in nature highlights its inherent stability. The balanced neutron-proton ratio within its nucleus contributes to this stability. Radioactive isotopes, such as ⁷Be and ¹⁰Be, offer valuable tools for scientific research. Their decay characteristics allow scientists to determine the ages of materials, track environmental changes, and investigate various natural processes.
The differing stability of beryllium isotopes influences their applications. The stability of ⁹Be makes it suitable for use in various industrial applications, discussed later. The radioactive isotopes find applications in scientific dating methods and tracers.
Electron Configuration and Chemical Behavior
Beryllium's electron configuration ([He] 2s²) plays a critical role in defining its chemical behavior. The two electrons in the outer shell determine its valence, its bonding capacity. This configuration suggests beryllium tends to lose its two valence electrons to achieve a stable, noble gas configuration (like helium). This explains beryllium's tendency to form 2+ cations (Be²⁺).
Beryllium's relatively small atomic size and high ionization energy contribute to its unique reactivity. While it's considered an alkaline earth metal, it shows less reactivity compared to other alkaline earth metals. This is primarily due to the strong electrostatic attraction between the nucleus and its valence electrons.
Applications of Beryllium and its Isotopes
The properties of beryllium and its isotopes contribute to their use in a range of fields:
-
⁹Be in Aerospace: Its low density and high stiffness make it ideal for lightweight aerospace components like aircraft parts and spacecraft structures.
-
⁹Be in Nuclear Applications: Beryllium is a neutron reflector and moderator, used in nuclear reactors and neutron sources.
-
⁹Be in X-ray Technology: Beryllium's ability to transmit X-rays makes it essential in X-ray windows and detectors.
-
⁷Be and ¹⁰Be in Dating and Research: These radioactive isotopes are invaluable tools in dating geological samples, reconstructing past climate conditions, and studying environmental processes.
-
Beryllium in Electronics: Its unique properties are utilized in some specialized electronic applications.
-
Beryllium in Alloys: It is used to strengthen and improve the properties of various metal alloys.
However, it is vital to remember that beryllium is a toxic material and should be handled with extreme caution.
Safety Precautions When Handling Beryllium
The toxicity of beryllium is a significant concern. Inhalation of beryllium dust can lead to a chronic lung disease called berylliosis, a serious and sometimes fatal condition. Appropriate safety measures are essential when handling beryllium, including:
-
Adequate ventilation: Work environments must have effective ventilation systems to minimize exposure to beryllium dust.
-
Personal Protective Equipment (PPE): Respiratory protection, including respirators, is crucial to prevent inhalation. Appropriate gloves and other protective clothing should also be used.
-
Proper Handling Procedures: Strict protocols for handling and disposal of beryllium-containing materials must be followed.
Conclusion: A Multifaceted Element
Beryllium, despite its relatively small atomic number, presents a fascinating array of properties and applications. Understanding its atomic structure – the number of protons, neutrons, and electrons – is fundamental to grasping its behavior and its role in various technological applications. Its isotopes display a range of stability, contributing to diverse uses in scientific research and industrial processes. However, the inherent toxicity of beryllium underscores the importance of exercising strict safety precautions during any handling and use. Further research into beryllium continues to unlock its potential and address the challenges associated with its use. The careful consideration of both its benefits and its risks is essential for responsible and safe utilization of this remarkable element.
Latest Posts
Latest Posts
-
Why Is The Vacuole Larger In Plant Cells
Apr 02, 2025
-
How To Initialize A Tuple In Python
Apr 02, 2025
-
Find The Acceleration When The Velocity Is 0
Apr 02, 2025
-
Are Metals Solid At Room Temperature
Apr 02, 2025
-
Which Of The Following Statements Correctly Describes Gene Linkage
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Number Of Protons Neutrons And Electrons In Beryllium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
