What Is The Bond Order Of Li2
News Leon
Apr 01, 2025 · 5 min read
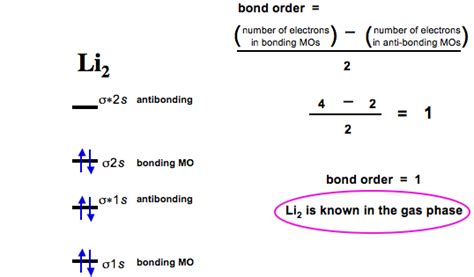
Table of Contents
What is the Bond Order of Li₂? A Deep Dive into Lithium's Molecular Orbital Theory
Determining the bond order of dilithium (Li₂) might seem like a simple task, but it provides a fantastic opportunity to delve into the fundamental concepts of molecular orbital (MO) theory. Understanding this seemingly simple molecule unlocks a deeper appreciation of chemical bonding and its predictive power. This article will thoroughly explore the calculation and implications of Li₂'s bond order, clarifying misconceptions and reinforcing key theoretical principles.
Understanding Bond Order: A Foundation
Before we tackle Li₂, let's solidify the concept of bond order. Simply put, bond order is the number of chemical bonds between a pair of atoms. It's a crucial indicator of the strength and stability of a chemical bond. A higher bond order generally translates to a stronger, shorter bond.
For homonuclear diatomic molecules (molecules composed of two atoms of the same element), like Li₂, we determine bond order using molecular orbital theory. This theory postulates that atomic orbitals combine to form molecular orbitals, which are occupied by electrons according to the Aufbau principle and Hund's rule.
The formula for bond order is straightforward:
Bond Order = (Number of electrons in bonding orbitals - Number of electrons in antibonding orbitals) / 2
Constructing the Molecular Orbital Diagram for Li₂
Lithium (Li) has three electrons: two in the 1s orbital and one in the 2s orbital. When two lithium atoms approach each other, their atomic orbitals interact to form molecular orbitals. The 1s orbitals combine to form a sigma (σ) bonding orbital (σ₁ₛ) and a sigma antibonding orbital (σ₁ₛ*). Similarly, the 2s orbitals combine to form a σ₂ₛ bonding orbital and a σ₂ₛ* antibonding orbital.
A simplified molecular orbital diagram for Li₂ would look like this:
Energy
σ₂ₛ* (Antibonding)
σ₂ₛ (Bonding)
σ₁ₛ* (Antibonding)
σ₁ₛ (Bonding)
Electron Configuration: Each lithium atom contributes three electrons to the molecular orbital system, resulting in a total of six electrons for Li₂. Following the Aufbau principle, these six electrons fill the molecular orbitals in order of increasing energy:
- Two electrons fill the σ₁ₛ orbital.
- Two electrons fill the σ₁ₛ* orbital.
- Two electrons fill the σ₂ₛ orbital.
Calculating the Bond Order of Li₂
Now, we can apply the bond order formula:
Bond Order = (Number of electrons in bonding orbitals - Number of electrons in antibonding orbitals) / 2
Bond Order = (4 - 2) / 2 = 1
Therefore, the bond order of Li₂ is 1. This indicates a single covalent bond between the two lithium atoms.
Implications of the Bond Order: Stability and Properties
A bond order of 1 implies a relatively weak bond compared to molecules with higher bond orders. This aligns with the observed properties of Li₂:
- Low bond dissociation energy: It requires relatively little energy to break the Li-Li bond.
- Long bond length: The Li-Li bond is relatively long due to the weak interaction.
- Reactivity: Li₂ is a highly reactive molecule, readily participating in chemical reactions to achieve a more stable electron configuration.
Comparing Li₂ to Other Alkali Metal Dimers
It's instructive to compare Li₂ to other alkali metal dimers like Na₂, K₂, Rb₂, and Cs₂. All these dimers exhibit single bonds (bond order of 1). However, the bond strength and length vary across the group, reflecting the changes in atomic size and effective nuclear charge. As we move down the group, the bond strength decreases, and the bond length increases. This trend is consistent with the increasing atomic size and decreasing effective nuclear charge.
Advanced Considerations: Beyond the Simplified Model
While the simplified MO diagram provides a good understanding of Li₂'s bonding, a more nuanced approach would consider the interactions between the 2s and 2p orbitals. In reality, the 2s and 2p orbitals are closer in energy than what is often depicted in simplified diagrams, leading to orbital mixing or hybridization. This mixing significantly influences the energies and shapes of the molecular orbitals, although the overall bond order remains 1.
Addressing Common Misconceptions
A common misconception is that the bond order is simply half the number of shared electrons. This simplification is not universally applicable, especially when considering antibonding orbitals. The formula explicitly accounts for the opposing effects of bonding and antibonding orbitals, providing a more accurate representation of bond strength.
Another misconception might stem from the fact that lithium readily loses its valence electron to form Li⁺ ion. However, in Li₂, the sharing of electrons leads to a more stable configuration than isolated lithium atoms, despite the bond's relative weakness.
The Importance of Molecular Orbital Theory
The study of Li₂, even with its seemingly simple structure, demonstrates the power and necessity of molecular orbital theory. This theory provides a robust framework for understanding chemical bonding beyond the limitations of valence bond theory, especially for molecules with multiple atoms and complex electron configurations.
Conclusion: Li₂ - A Stepping Stone to Deeper Understanding
Understanding the bond order of Li₂ is more than just a calculation; it’s a gateway to grasping fundamental principles of molecular orbital theory. By examining this seemingly simple diatomic molecule, we can appreciate the complexities and intricacies of chemical bonding and its impact on the properties and reactivity of molecules. The concepts explored here are fundamental to advanced studies in chemistry, providing a solid base for understanding more complex chemical systems. The relatively weak bond in Li₂, resulting in a bond order of 1, is a significant finding that underscores the importance of considering both bonding and antibonding orbitals when evaluating molecular stability and reactivity. Furthermore, comparing Li₂ to other alkali metal dimers highlights the periodic trends in bonding strength and provides a deeper understanding of the relationship between atomic structure and molecular properties.
Latest Posts
Latest Posts
-
What Is The Best Topic For Speech
Apr 02, 2025
-
Why Is The Vacuole Larger In Plant Cells
Apr 02, 2025
-
How To Initialize A Tuple In Python
Apr 02, 2025
-
Find The Acceleration When The Velocity Is 0
Apr 02, 2025
-
Are Metals Solid At Room Temperature
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Bond Order Of Li2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
