What Is The Bond Order Of O2
News Leon
Mar 20, 2025 · 5 min read
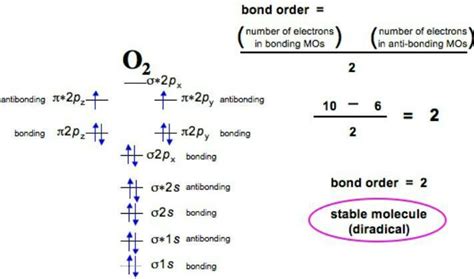
Table of Contents
What is the Bond Order of O2? A Deep Dive into Molecular Orbital Theory
Determining the bond order of a molecule is crucial for understanding its stability and properties. Oxygen (O₂), a vital component of our atmosphere and essential for life, presents a fascinating case study in molecular orbital theory (MOT). This article will delve deep into the intricacies of O₂'s electronic structure, ultimately explaining how its bond order is calculated and what implications this has for its reactivity and characteristics.
Understanding Molecular Orbital Theory
Before we tackle the bond order of O₂, let's establish a firm understanding of the theoretical framework underlying our calculations: Molecular Orbital Theory. Unlike Valence Bond Theory, which focuses on localized bonds, MOT considers the combination of atomic orbitals to form molecular orbitals that encompass the entire molecule.
Atomic Orbitals vs. Molecular Orbitals
Each oxygen atom possesses eight electrons arranged in the electronic configuration 1s²2s²2p⁴. When two oxygen atoms approach each other, their atomic orbitals interact, leading to the formation of molecular orbitals. These molecular orbitals are classified as either bonding or antibonding, depending on their energy levels and electron distribution.
Constructing Molecular Orbitals for O₂
The combination of atomic orbitals in O₂ follows specific rules dictated by symmetry and energy considerations. The 1s and 2s atomic orbitals interact to form σ and σ* molecular orbitals (sigma and sigma-star), respectively. The 2p atomic orbitals, which consist of three orbitals (2px, 2py, and 2pz), give rise to a more complex set of molecular orbitals.
- σ2p and σ*2p: The 2pz orbitals (oriented along the internuclear axis) interact to form a bonding σ2p and an antibonding σ*2p molecular orbital.
- π2p and π*2p: The 2px and 2py orbitals (perpendicular to the internuclear axis) combine to form two degenerate bonding π2p molecular orbitals and two degenerate antibonding π*2p molecular orbitals. Degenerate means they have the same energy level.
Filling the Molecular Orbitals
With a total of 16 electrons (8 from each oxygen atom), we fill the molecular orbitals according to the Aufbau principle (filling lower energy levels first) and Hund's rule (maximizing electron spin before pairing). The order of filling is: σ2s, σ2s, σ2p, π2p, π2p, σ*2p.
Electron Configuration of O₂: (σ2s)²(σ2s)²(σ2p)²(π2p)⁴(π2p)²
Calculating the Bond Order of O₂
The bond order is a critical parameter that reflects the strength and stability of a chemical bond. It's defined as half the difference between the number of electrons in bonding orbitals and the number of electrons in antibonding orbitals.
Bond Order = (Number of electrons in bonding orbitals - Number of electrons in antibonding orbitals) / 2
Let's apply this to O₂:
- Number of electrons in bonding orbitals: 8 (2 from σ2s, 2 from σ2p, 4 from π2p)
- Number of electrons in antibonding orbitals: 4 (2 from σ2s, 2 from π2p)
- Bond Order: (8 - 4) / 2 = 2
Therefore, the bond order of O₂ is 2. This indicates a double bond between the two oxygen atoms, explaining the relative stability of the O₂ molecule.
Implications of O₂'s Bond Order
The bond order of 2 has significant implications for the properties and reactivity of oxygen:
Paramagnetism
The presence of two unpaired electrons in the degenerate π2p orbitals makes O₂ paramagnetic, meaning it is weakly attracted to a magnetic field. This is a unique property that contrasts with most other diatomic molecules with an even number of electrons. This paramagnetism is a direct consequence of the molecular orbital configuration and the incompletely filled π2p orbitals.
Bond Length and Strength
A bond order of 2 reflects a relatively strong double bond. This double bond contributes to a shorter bond length and higher bond dissociation energy compared to a single bond. This strength is crucial for the stability of the O₂ molecule and its role in various chemical reactions.
Reactivity
While relatively stable, O₂ is still a highly reactive molecule. The presence of unpaired electrons in the π*2p orbitals makes it susceptible to oxidation reactions. This high reactivity is essential for its role in respiration and various combustion processes.
Comparing O₂ with Other Diatomic Molecules
Comparing O₂ to other diatomic molecules helps illustrate the significance of its bond order and electronic structure. For example:
- N₂ (Nitrogen): Nitrogen has a triple bond (bond order of 3) due to its electronic configuration. This results in an extremely strong and stable molecule, significantly less reactive than O₂.
- F₂ (Fluorine): Fluorine has a single bond (bond order of 1), making it more reactive than O₂ because of its weaker bond strength.
This comparison highlights how the bond order, dictated by the molecular orbital configuration, influences a molecule's properties.
Advanced Concepts and Further Exploration
This exploration of O₂'s bond order touches upon fundamental aspects of MOT. However, a deeper understanding requires exploring more complex aspects:
Spin states
The presence of unpaired electrons in O₂ leads to multiple spin states, affecting its reactivity and spectroscopic properties. A detailed analysis of these spin states requires a more advanced treatment of molecular orbital theory.
Influence of Interatomic Distance
The bond order isn't a fixed value; it can change subtly with variations in interatomic distance. These subtle changes are important in understanding dynamic processes such as vibrational spectroscopy.
Computational Chemistry
Computational chemistry offers powerful tools to simulate molecular orbitals and calculate bond orders with high accuracy. These methods allow for detailed investigation of O₂'s electronic structure and its interactions with other molecules.
Conclusion
The bond order of O₂ is a fundamental characteristic determined by its molecular orbital configuration. The value of 2, arising from eight bonding and four antibonding electrons, accurately reflects the double bond between the oxygen atoms. This double bond, along with the presence of unpaired electrons in the antibonding orbitals, dictates its paramagnetism and reactivity—properties that are vital for its role in numerous chemical and biological processes. A comprehensive understanding of O₂'s bond order and electronic structure is crucial for appreciating its fundamental importance in chemistry and beyond. Further exploration of molecular orbital theory reveals a deeper appreciation for the complexity and elegance of chemical bonding.
Latest Posts
Latest Posts
-
What Is The Molecular Mass Of Ccl4
Mar 20, 2025
-
Activation Energy For The Forward Reaction
Mar 20, 2025
-
Under What Conditions Are Gases Most Likely To Behave Ideally
Mar 20, 2025
-
Which Of The Following Is A Non Phagocytic Cell
Mar 20, 2025
-
Ball A Of Mass 5 0 Kilograms
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Bond Order Of O2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
