Activation Energy For The Forward Reaction
News Leon
Mar 20, 2025 · 6 min read
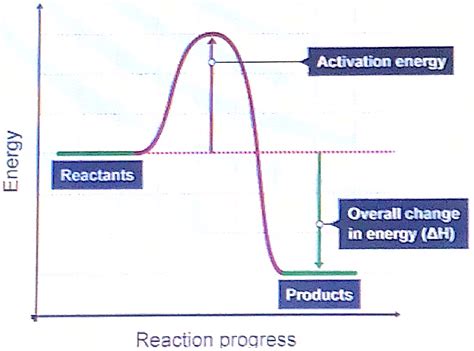
Table of Contents
Activation Energy for the Forward Reaction: A Deep Dive
Understanding chemical reactions and their rates is crucial in numerous fields, from industrial chemistry and materials science to biology and environmental science. A key concept governing reaction rates is activation energy, specifically the activation energy for the forward reaction. This article delves into the intricacies of activation energy, exploring its definition, significance, factors influencing it, and its practical applications. We'll cover how activation energy relates to reaction rate constants, explore the Arrhenius equation, and discuss methods for determining activation energy experimentally.
What is Activation Energy?
Activation energy (Ea) is the minimum amount of energy required for a chemical reaction to occur. It's the energy barrier that reactant molecules must overcome to transform into products. Think of it like pushing a boulder up a hill: the energy required to get the boulder to the top of the hill represents the activation energy. Once the boulder reaches the top, it can roll down the other side, representing the formation of products.
Crucially, activation energy is specific to the forward reaction. The reverse reaction, converting products back to reactants, will have its own, potentially different, activation energy. This difference is significant because it reflects the energy landscape of the reaction.
The Transition State
Reactant molecules don't simply transform into products instantaneously. They first form a transition state (also called the activated complex), a high-energy, unstable intermediate structure. The transition state represents the point of maximum energy along the reaction pathway. The energy difference between the reactants and the transition state is the activation energy for the forward reaction.
Factors Affecting Activation Energy
Several factors can influence the activation energy of a forward reaction:
1. Nature of Reactants:
The inherent chemical properties of the reactants significantly impact activation energy. Strong bonds require more energy to break than weak bonds. For example, reactions involving the breaking of multiple strong covalent bonds (e.g., C-C or C-H bonds) will generally have higher activation energies than reactions involving weaker bonds (e.g., hydrogen bonds). The electronic structure and reactivity of reactants play a pivotal role.
2. Reaction Mechanism:
The mechanism of a reaction—the series of elementary steps involved—directly affects the activation energy. A reaction proceeding through a multi-step mechanism will have an overall activation energy determined by the highest-energy transition state along the reaction pathway (the rate-determining step). A simple, one-step mechanism usually has a lower activation energy than a complex, multi-step one.
3. Temperature:
While temperature doesn't directly change the activation energy itself, it profoundly impacts the fraction of molecules possessing sufficient energy to overcome the activation energy barrier. Higher temperatures lead to a larger fraction of molecules with energies exceeding Ea, thus accelerating the reaction rate. This relationship is elegantly captured by the Arrhenius equation.
4. Catalysts:
Catalysts are substances that increase the rate of a reaction without being consumed themselves. They achieve this by providing an alternative reaction pathway with a lower activation energy. Catalysts often work by binding to reactants, stabilizing the transition state, or facilitating bond breaking and formation through intermediate complexes. Enzyme catalysis in biological systems provides a compelling example of this effect.
5. Surface Area (for Heterogeneous Reactions):
In heterogeneous reactions (reactions occurring at the interface between two phases, such as a solid and a gas), the surface area of the solid reactant significantly affects the activation energy. A larger surface area provides more sites for reaction to occur, effectively lowering the activation energy and speeding up the reaction.
The Arrhenius Equation: Connecting Activation Energy and Reaction Rate
The Arrhenius equation mathematically relates the rate constant (k) of a reaction to the activation energy (Ea), the temperature (T), and the pre-exponential factor (A):
k = A * exp(-Ea/RT)
Where:
- k is the rate constant
- A is the pre-exponential factor (frequency factor), representing the frequency of collisions between reactant molecules with the correct orientation.
- Ea is the activation energy
- R is the ideal gas constant
- T is the absolute temperature (in Kelvin)
This equation highlights the exponential dependence of the rate constant on activation energy and temperature. A lower activation energy leads to a higher rate constant, and a higher temperature leads to a faster reaction rate.
The Arrhenius equation can be linearized by taking the natural logarithm of both sides:
ln(k) = ln(A) - Ea/RT
This linear form is useful for determining activation energy experimentally. By plotting ln(k) versus 1/T, the slope of the resulting straight line is equal to -Ea/R, allowing for the calculation of Ea.
Experimental Determination of Activation Energy
Several experimental techniques can be employed to determine the activation energy for a forward reaction:
1. The Arrhenius Plot Method:
As described above, this involves measuring the rate constant (k) at several different temperatures. Plotting ln(k) against 1/T yields a straight line with a slope of -Ea/R.
2. Differential Method:
This method utilizes the integrated rate law for the specific reaction order. By measuring the change in concentration over time at different temperatures, one can determine the rate constant at each temperature and then use the Arrhenius equation to calculate the activation energy.
3. Spectroscopic Methods:
Techniques such as UV-Vis spectroscopy or infrared spectroscopy can monitor the concentration of reactants and products over time, providing data for determining the rate constant and subsequently the activation energy.
4. Computational Methods:
Advanced computational chemistry techniques, such as density functional theory (DFT), can be used to calculate activation energies theoretically. These methods provide valuable insights into the reaction mechanism and the nature of the transition state.
Significance and Applications of Activation Energy
Understanding activation energy has widespread implications across various scientific disciplines:
-
Catalysis: Designing efficient catalysts hinges on minimizing activation energy. Researchers continuously strive to develop catalysts that lower activation energies for desired reactions, enhancing reaction rates and improving efficiency in industrial processes and biological systems.
-
Reaction Kinetics: Activation energy is central to understanding and predicting the rates of chemical reactions. Knowledge of activation energy allows scientists to model reaction kinetics and optimize reaction conditions for desired outcomes.
-
Chemical Engineering: In chemical engineering, activation energy data is critical for designing and scaling up chemical processes, ensuring efficient and safe operation of reactors.
-
Materials Science: Activation energy plays a role in understanding the kinetics of material synthesis, degradation, and phase transformations. Controlling activation energy is crucial in designing materials with tailored properties.
-
Biology: Enzyme-catalyzed reactions in living organisms heavily rely on activation energy reduction. The efficiency and specificity of enzymatic catalysis are directly related to the lowering of activation energy for biological processes.
-
Environmental Science: Activation energy is relevant in studying environmental processes such as atmospheric reactions and pollutant degradation. Understanding activation energies helps model pollutant transformation rates and predict their environmental impact.
Conclusion
Activation energy for the forward reaction is a fundamental concept governing the rate at which chemical reactions proceed. Its influence extends across numerous fields, impacting industrial processes, material design, biological systems, and environmental studies. The ability to determine and manipulate activation energy is critical for optimizing chemical reactions, designing effective catalysts, and understanding complex chemical processes. Further advancements in understanding and controlling activation energy will continue to drive innovation across various scientific and technological domains. The Arrhenius equation provides a powerful tool for relating activation energy to reaction rates, and various experimental and computational methods enable the determination of activation energy values. A deep understanding of this essential concept is crucial for anyone working in chemistry, materials science, or related fields.
Latest Posts
Latest Posts
-
Which Of The Following Are Characteristics Of Prokaryotes
Mar 21, 2025
-
How To Calculate The Emf Of A Battery
Mar 21, 2025
-
A Rock Is Thrown Vertically Upward From Ground Level
Mar 21, 2025
-
3 14 X 6 To The Power Of 2
Mar 21, 2025
-
If Cells Are Placed In A Hypertonic Solution
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Activation Energy For The Forward Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
