If Cells Are Placed In A Hypertonic Solution
News Leon
Mar 21, 2025 · 7 min read
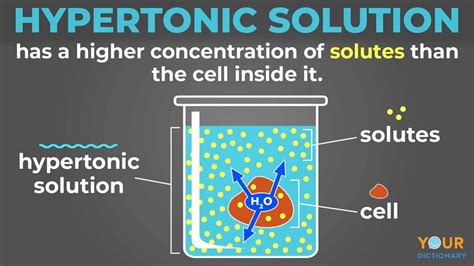
Table of Contents
What Happens When Cells Are Placed in a Hypertonic Solution?
Cells are the fundamental units of life, and their interaction with their surrounding environment is crucial for their survival and function. One important aspect of this interaction involves the concept of tonicity, specifically, what happens when cells are placed in a hypertonic solution. Understanding this process is vital in various fields, from medicine and biology to agriculture and environmental science. This comprehensive article will explore the effects of hypertonic solutions on different types of cells, the underlying mechanisms, and the implications of this phenomenon.
Understanding Tonicity: Isotonic, Hypotonic, and Hypertonic Solutions
Before diving into the specifics of hypertonic solutions, let's establish a clear understanding of tonicity. Tonicity describes the relative concentration of solutes in two solutions separated by a selectively permeable membrane, such as a cell membrane. This comparison focuses on the water potential or the tendency of water to move across the membrane. There are three main types of tonicity:
-
Isotonic Solution: In an isotonic solution, the concentration of solutes is equal both inside and outside the cell. There is no net movement of water across the cell membrane; water moves in and out at an equal rate, maintaining cell volume.
-
Hypotonic Solution: A hypotonic solution has a lower concentration of solutes compared to the inside of the cell. This means there is a higher concentration of water outside the cell. Water moves into the cell by osmosis, causing the cell to swell and potentially burst (lyse) if the influx of water is excessive.
-
Hypertonic Solution: A hypertonic solution has a higher concentration of solutes compared to the inside of the cell. This implies a lower concentration of water outside the cell. As a result, water moves out of the cell by osmosis, causing the cell to shrink or crenate.
The Effects of Hypertonic Solutions on Animal Cells
When animal cells are placed in a hypertonic solution, water rushes out of the cell due to osmosis. This outward movement of water leads to several consequences:
-
Cell shrinkage or crenation: The most prominent effect is the reduction in cell volume. The cell membrane pulls away from the cell wall (if present), resulting in a shrunken, wrinkled appearance. This process is known as crenation. The degree of crenation depends on the concentration of the hypertonic solution and the duration of exposure.
-
Plasma membrane disruption: Severe dehydration caused by prolonged exposure to a highly concentrated hypertonic solution can damage the cell membrane's integrity. This can lead to compromised cell function and eventually cell death.
-
Changes in cellular metabolism: The loss of water affects the cellular environment, impacting the function of various cellular components and enzymes. This can result in decreased metabolic activity and ultimately cell dysfunction.
-
Alteration of cell signaling: Cell-to-cell communication and signaling pathways can be disrupted by the changes in cell volume and osmotic pressure, leading to a cascade of downstream effects.
-
Cell death (apoptosis or necrosis): If the cell is unable to adapt to the drastic change in water potential, it may undergo programmed cell death (apoptosis) or uncontrolled cell death (necrosis). The specific mechanism of cell death depends on the severity and duration of hypertonic stress.
The Effects of Hypertonic Solutions on Plant Cells
Plant cells differ from animal cells in that they possess a rigid cell wall. This cell wall provides structural support and protection. When placed in a hypertonic solution, plant cells respond differently:
-
Plasmolysis: The outward movement of water causes the cell membrane to pull away from the cell wall. This process is called plasmolysis. The cell shrinks, but its shape is largely maintained due to the rigid cell wall. The space between the cell membrane and the cell wall becomes visible.
-
Reduced turgor pressure: Turgor pressure is the pressure exerted by the cell contents against the cell wall. This pressure is essential for maintaining plant cell rigidity and overall plant structure. In a hypertonic solution, turgor pressure decreases significantly, leading to wilting.
-
Inhibition of growth: Plasmolysis and reduced turgor pressure inhibit cell growth and expansion. This can have significant implications for plant development and overall health.
-
Reversibility (under certain conditions): Unlike animal cells, plant cells can often recover from plasmolysis if they are transferred back to an isotonic or hypotonic solution. The cell membrane can reattach to the cell wall, and turgor pressure is restored. However, prolonged exposure to a hypertonic solution can cause irreversible damage.
Mechanisms Underlying Osmosis in Hypertonic Solutions
The movement of water across cell membranes in hypertonic solutions is governed by the principles of osmosis. Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement continues until equilibrium is reached, or the water potential is equalized on both sides of the membrane.
The driving force behind osmosis is the water potential gradient. Water potential is the measure of the free energy of water, and it's influenced by solute concentration, pressure, and gravity. Water always moves from an area of higher water potential to an area of lower water potential. In a hypertonic solution, the water potential outside the cell is lower than inside the cell, causing water to move out of the cell.
Applications and Significance
The effects of hypertonic solutions have several important applications and implications in various fields:
-
Food preservation: Hypertonic solutions are used in food preservation techniques, such as pickling and salting. The high solute concentration in these solutions draws water out of microorganisms, inhibiting their growth and preventing spoilage.
-
Medicine: Hypertonic solutions are used in intravenous (IV) fluids for specific medical conditions, although carefully controlled. They can help to reduce edema (swelling) by drawing fluid from tissues into the bloodstream. However, improper use can lead to serious complications.
-
Agriculture: Understanding the effects of hypertonic solutions is crucial in agriculture for managing soil salinity. High salt concentrations in soil can cause plasmolysis in plant cells, leading to reduced growth and crop yield.
-
Environmental science: Hypertonic conditions can occur in various environmental settings, such as estuaries and salt marshes. Organisms living in these environments have adapted to tolerate fluctuating osmotic conditions. Studying these adaptations is essential for understanding ecosystem dynamics.
-
Cell biology research: Hypertonic solutions are used extensively in cell biology research to study cell responses to osmotic stress and to investigate various cellular processes. They are useful tools for manipulating cell volume and studying the effects on cell function.
Factors Affecting the Response to Hypertonic Solutions
Several factors can influence the extent to which a cell is affected by a hypertonic solution:
-
Concentration of the solution: The higher the concentration of solutes in the hypertonic solution, the greater the osmotic pressure and the more significant the water loss from the cell.
-
Duration of exposure: Prolonged exposure to a hypertonic solution leads to more severe effects compared to brief exposure.
-
Type of cell: Different types of cells have varying tolerance to hypertonic conditions. Some cells are more resistant to osmotic stress than others.
-
Cell wall (in plant cells): The presence of a cell wall in plant cells significantly modifies the response to hypertonic solutions, as it prevents complete cell lysis.
-
Aquaporins: Aquaporins are water channels in cell membranes that facilitate water transport. The number and activity of aquaporins can influence the rate of water movement in and out of the cell during osmotic stress.
Conclusion
The effects of placing cells in a hypertonic solution are profound and far-reaching. Understanding the principles of osmosis and the specific responses of different cell types is crucial in many scientific disciplines. While hypertonic solutions can be harmful, causing cell shrinkage and even death, they also have important applications in diverse fields like food preservation and medicine. Further research into cellular mechanisms related to osmotic stress will continue to expand our understanding of cell biology and its implications in various aspects of life. This knowledge is essential for developing effective strategies to mitigate the negative impacts of hypertonic environments and harnessing the beneficial applications of hypertonic solutions.
Latest Posts
Latest Posts
-
Definition Of Average Acceleration In Physics
Mar 21, 2025
-
Opportunity Cost Occurs Because Of A Producers Need To
Mar 21, 2025
-
How Many Chambers Does A Frogs Heart Have
Mar 21, 2025
-
Is Burning Of Paper A Chemical Change
Mar 21, 2025
-
Which Of The Following Is Not A Money Market Security
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about If Cells Are Placed In A Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
