Under What Conditions Are Gases Most Likely To Behave Ideally
News Leon
Mar 20, 2025 · 5 min read
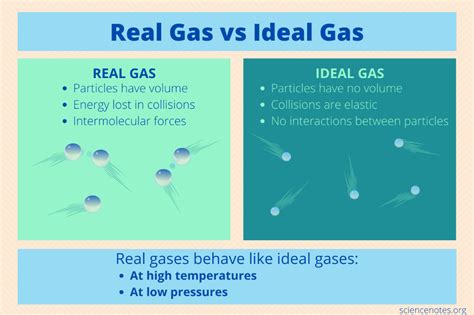
Table of Contents
Under What Conditions Are Gases Most Likely to Behave Ideally?
Understanding the behavior of gases is crucial in various scientific fields, from chemistry and physics to engineering and meteorology. While the Ideal Gas Law provides a simplified model, real gases deviate from this ideal behavior under certain conditions. This article delves deep into the conditions under which gases are most likely to exhibit ideal behavior, exploring the factors that contribute to deviations and the underlying principles of the kinetic molecular theory.
The Ideal Gas Law: A Foundation
The Ideal Gas Law, expressed as PV = nRT, describes the relationship between pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). This law assumes that gas particles are point masses with negligible volume and that there are no intermolecular forces between them. These assumptions, while simplifying calculations, are not entirely accurate for real gases.
Assumptions of the Ideal Gas Law:
-
Negligible Volume of Gas Molecules: Ideal gas molecules are considered point masses with no volume. In reality, gas molecules do occupy a finite volume, and at high pressures, this volume becomes significant compared to the total volume of the container.
-
No Intermolecular Forces: Ideal gases are assumed to have no attractive or repulsive forces between molecules. However, real gases experience various intermolecular forces, such as van der Waals forces (London dispersion forces, dipole-dipole interactions, and hydrogen bonding), which significantly affect their behavior, especially at low temperatures and high pressures.
-
Elastic Collisions: Collisions between gas molecules and the container walls are assumed to be perfectly elastic, meaning no kinetic energy is lost during collisions. In reality, some energy is lost as heat during collisions, although this effect is often negligible.
-
Random Motion: Gas molecules are assumed to move randomly and independently. This assumption works well for most gases under normal conditions.
Deviations from Ideal Behavior: Understanding Real Gases
Real gases deviate from the Ideal Gas Law because the assumptions of the law are not entirely accurate. The magnitude of these deviations depends on several factors.
High Pressure: The Role of Molecular Volume
At high pressures, gas molecules are compressed into a smaller volume. The volume occupied by the gas molecules themselves becomes a significant fraction of the total volume. This effectively reduces the available space for the molecules to move, leading to a larger pressure than predicted by the Ideal Gas Law. The compressibility factor (Z = PV/nRT) becomes greater than 1 under high pressure conditions.
Low Temperature: The Impact of Intermolecular Forces
At low temperatures, the kinetic energy of the gas molecules decreases. This makes the attractive intermolecular forces more significant compared to the kinetic energy. These forces cause the gas molecules to clump together, reducing the effective number of particles that collide with the container walls and resulting in a lower pressure than predicted by the Ideal Gas Law. The compressibility factor becomes less than 1 under low temperature conditions.
Type of Gas: The Influence of Molecular Structure
The type of gas plays a crucial role in determining its deviation from ideality. Gases with larger, more complex molecules tend to deviate more significantly than smaller, simpler gases. This is because larger molecules have a greater volume and stronger intermolecular forces. For example, polar molecules exhibit stronger dipole-dipole interactions compared to nonpolar molecules, leading to greater deviations.
Polarity and Molecular Weight: Amplifying Deviations
Polar molecules possess permanent dipoles, resulting in stronger intermolecular attractions compared to nonpolar molecules. These attractions cause greater deviations from ideal behavior, especially at lower temperatures. Similarly, heavier gases with higher molecular weights tend to have stronger London dispersion forces, leading to more pronounced deviations from ideality.
Conditions Favoring Ideal Gas Behavior
Considering the factors contributing to deviations, we can identify conditions under which gases are most likely to behave ideally:
High Temperature and Low Pressure: The Optimal Combination
The most crucial factors influencing ideal behavior are temperature and pressure. High temperatures provide gas molecules with sufficient kinetic energy to overcome intermolecular attractive forces. This minimizes the impact of these forces on the gas's behavior. Low pressures ensure that the volume occupied by the gas molecules themselves is negligible compared to the total volume of the container.
Under these conditions, the assumptions of the Ideal Gas Law—negligible molecular volume and negligible intermolecular forces—are most closely approximated.
Simple Molecular Structure: Minimizing Interactions
Gases composed of small, nonpolar molecules, such as helium (He), neon (Ne), and argon (Ar), exhibit near-ideal behavior over a wider range of conditions compared to gases with larger, more complex molecules. This is because simple molecules have weaker intermolecular forces and smaller molecular volumes.
The Compressibility Factor: A Quantitative Measure of Ideality
The compressibility factor (Z) provides a quantitative measure of how much a real gas deviates from ideal behavior. It is defined as:
Z = PV/nRT
For an ideal gas, Z = 1. Deviations from Z = 1 indicate non-ideal behavior. A Z value greater than 1 suggests that repulsive forces dominate, while a Z value less than 1 suggests that attractive forces are more prominent.
Beyond the Ideal Gas Law: More Realistic Models
Several equations of state, such as the van der Waals equation, have been developed to account for the deviations of real gases from ideal behavior. These equations introduce correction factors to account for the finite volume of gas molecules and the presence of intermolecular forces.
Conclusion: A Practical Perspective
While the Ideal Gas Law provides a valuable simplification, it's essential to understand the conditions under which it breaks down. Gases behave most ideally at high temperatures and low pressures, particularly for gases with simple, nonpolar molecules. This knowledge is crucial for accurate predictions and calculations involving gases in various applications. Understanding deviations from ideality and employing more sophisticated models like the van der Waals equation is vital for accurately describing the behavior of real gases in scenarios where the Ideal Gas Law falls short. By understanding these factors, scientists and engineers can accurately model and predict the behavior of gases in various applications and design systems that operate efficiently and safely. The compressibility factor serves as a valuable tool for assessing the degree of ideality and selecting the appropriate model for a given situation.
Latest Posts
Latest Posts
-
Is Glycogen A Monosaccharide Disaccharide Or Polysaccharide
Mar 21, 2025
-
C2h6 O2 Co2 H2o
Mar 21, 2025
-
How Many Total Electrons Does Carbon Have
Mar 21, 2025
-
Which Of The Following Is Not A Membranous Organelle
Mar 21, 2025
-
The Space Between The Cornea And The Iris Is The
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Under What Conditions Are Gases Most Likely To Behave Ideally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
