How Many Total Electrons Does Carbon Have
News Leon
Mar 21, 2025 · 5 min read
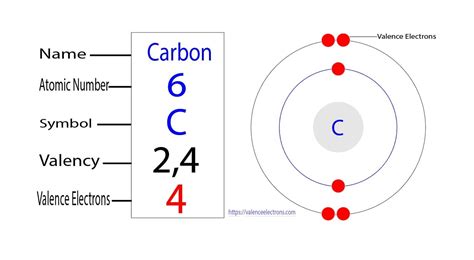
Table of Contents
How Many Total Electrons Does Carbon Have? A Deep Dive into Atomic Structure
Carbon, the cornerstone of organic chemistry and the building block of life as we know it, holds a fascinating position in the periodic table. Understanding its electron configuration is key to grasping its remarkable versatility and ability to form an incredibly diverse range of molecules. So, how many electrons does a carbon atom possess? The answer, simply put, is six. But let's delve deeper into the "why" and explore the implications of this seemingly simple number.
Understanding Atomic Structure and Electron Shells
Before we pinpoint the number of electrons in carbon, let's briefly review the fundamentals of atomic structure. An atom consists of a central nucleus containing positively charged protons and neutral neutrons. Surrounding this nucleus is a cloud of negatively charged electrons, orbiting in specific energy levels or shells. These shells are crucial because they dictate how an atom interacts with other atoms to form chemical bonds.
The number of protons in an atom's nucleus defines its atomic number and determines its identity as a specific element. In a neutral atom, the number of electrons equals the number of protons. This balance of positive and negative charges ensures overall electrical neutrality.
Carbon's Atomic Structure: A Closer Look
Carbon (C) has an atomic number of 6. This means a neutral carbon atom possesses six protons in its nucleus. Therefore, to maintain electrical neutrality, it also has six electrons.
These six electrons are distributed across two electron shells:
- Shell 1 (K shell): This innermost shell can hold a maximum of two electrons. In a carbon atom, this shell is completely filled with two electrons.
- Shell 2 (L shell): This outer shell can accommodate up to eight electrons. In carbon, the remaining four electrons reside in this shell. It's this outer shell, often referred to as the valence shell, that determines carbon's chemical reactivity and bonding behavior.
Carbon's Valence Electrons: The Key to Chemical Bonding
The electrons in the outermost shell, the valence electrons, are responsible for the chemical properties of an element. Carbon's four valence electrons are the reason behind its exceptional ability to form strong covalent bonds with various other atoms. These bonds involve the sharing of electron pairs between atoms, resulting in stable molecules.
This tetravalency, the capacity to form four bonds, distinguishes carbon from most other elements. This unique characteristic is responsible for the vast diversity and complexity of organic molecules, which are the foundation of life.
The Importance of Carbon's Electron Configuration in Organic Chemistry
Carbon's electron configuration has profound implications for the field of organic chemistry. The four valence electrons allow carbon atoms to form long chains, branched structures, and rings – the building blocks of a huge array of organic compounds. This ability to form strong, stable bonds with other carbon atoms and various other elements (like hydrogen, oxygen, nitrogen, and sulfur) is the basis for the vast complexity of organic molecules found in living organisms and synthetic materials.
Examples include:
- Hydrocarbons: These are composed solely of carbon and hydrogen atoms, forming the backbone of many fuels and plastics (e.g., methane, ethane, propane, polyethylene). The strong carbon-carbon and carbon-hydrogen bonds contribute to their stability.
- Carbohydrates: These essential biological molecules, such as sugars and starches, contain carbon, hydrogen, and oxygen. Their structures are based on carbon chains with attached hydroxyl (-OH) groups.
- Proteins: These complex biomolecules are crucial for structural support, catalysis, and many other biological functions. Their fundamental building blocks, amino acids, all contain a central carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a side chain (R group) that varies among different amino acids.
- Lipids (fats and oils): These are essential components of cell membranes and energy storage. Their structures often involve long hydrocarbon chains, again showcasing carbon's ability to form extensive chains.
- Nucleic Acids (DNA and RNA): These molecules carry genetic information and are vital for inheritance and protein synthesis. Their structures rely heavily on carbon-based backbones and nitrogenous bases.
Each of these classes of biomolecules possesses unique properties directly attributable to the specific arrangement of carbon atoms and their bonding to other atoms, all stemming from carbon's four valence electrons.
Carbon's Isotopes and Electron Count
While a neutral carbon atom always has six electrons, it's important to note that carbon exists in several isotopic forms. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The most common isotopes of carbon are carbon-12 (¹²C) and carbon-13 (¹³C), both with six electrons. Carbon-14 (¹⁴C), a radioactive isotope, is used in radiocarbon dating, and it too has six electrons. The number of neutrons affects the atom's mass but not its electron count or its chemical properties.
Carbon's Role in Various Fields
Beyond its central role in organic chemistry and biochemistry, carbon plays a crucial role in many other scientific and technological fields:
- Materials Science: Carbon is a key component in many advanced materials, including graphite, diamond, and fullerenes (like buckminsterfullerene or "buckyballs"). The different allotropes of carbon exhibit diverse properties due to variations in their atomic arrangements, but the electron count remains constant.
- Nanotechnology: Carbon nanotubes and graphene, derived from carbon, have exceptional mechanical, electrical, and thermal properties, leading to numerous applications in electronics, materials science, and medicine.
- Environmental Science: Carbon's role in the carbon cycle, the process by which carbon atoms are exchanged between various reservoirs (atmosphere, oceans, land), is essential for understanding climate change.
Conclusion: The Significance of Six Electrons
The seemingly simple answer – six electrons – belies the immense importance of carbon's electron configuration. Its four valence electrons enable the formation of a vast array of molecules, forming the basis for the incredible diversity of organic compounds, the building blocks of life, and numerous advanced materials. Understanding the structure of the carbon atom and its electron distribution is fundamental to grasping the complexity and importance of this element in various fields of science and technology. The seemingly small number, six, signifies a massive contribution to the world around us, from the smallest living organism to the most advanced technology.
Latest Posts
Latest Posts
-
Bundles Of Axons In The Central Nervous System Are Called
Mar 21, 2025
-
How To Find Average Velocity On A Velocity Time Graph
Mar 21, 2025
-
Which Of The Following Is Not An Example Of
Mar 21, 2025
-
Why Water Is Liquid At Room Temperature
Mar 21, 2025
-
In Which Organ Does Fermentation Begin To Occur
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Many Total Electrons Does Carbon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
