What Is The Molecular Mass Of Ccl4
News Leon
Mar 20, 2025 · 5 min read
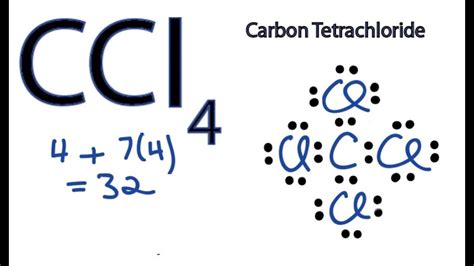
Table of Contents
What is the Molecular Mass of CCl4? A Deep Dive into Carbon Tetrachloride
Carbon tetrachloride (CCl₄), also known as tetrachloromethane, is a colorless, volatile, and dense liquid with a characteristic ethereal odor. While its uses have diminished due to its toxicity, understanding its properties, particularly its molecular mass, remains crucial in various scientific and industrial contexts. This article will delve into the calculation and significance of the molecular mass of CCl₄, exploring related concepts and applications.
Understanding Molecular Mass
Before calculating the molecular mass of CCl₄, let's establish a clear understanding of what molecular mass represents. Molecular mass, also known as molecular weight, is the mass of a molecule. It's expressed in atomic mass units (amu) or Daltons (Da). It's calculated by summing the atomic masses of all the atoms present in a single molecule of a compound. This sum considers the number of atoms of each element present in the molecule.
Atomic Mass: The Building Blocks
The foundation of molecular mass calculation lies in the atomic mass of each element. Atomic mass represents the average mass of an atom of an element, taking into account the different isotopes of that element and their relative abundances. These values are readily available in the periodic table of elements. For example, the atomic mass of carbon (C) is approximately 12.01 amu, while the atomic mass of chlorine (Cl) is approximately 35.45 amu.
Calculating the Molecular Mass of CCl₄
Now, let's calculate the molecular mass of CCl₄:
-
Identify the elements and their number: One molecule of CCl₄ contains one carbon atom (C) and four chlorine atoms (Cl).
-
Find the atomic mass of each element: From the periodic table:
- Atomic mass of Carbon (C) ≈ 12.01 amu
- Atomic mass of Chlorine (Cl) ≈ 35.45 amu
-
Calculate the total mass:
- Mass of Carbon: 1 C atom × 12.01 amu/C atom = 12.01 amu
- Mass of Chlorine: 4 Cl atoms × 35.45 amu/Cl atom = 141.80 amu
- Total Molecular Mass: 12.01 amu + 141.80 amu = 153.81 amu
Therefore, the molecular mass of CCl₄ is approximately 153.81 amu.
The Significance of Molecular Mass in Various Applications
The molecular mass of CCl₄, and molecular mass in general, is a crucial parameter in numerous scientific and industrial applications. Its significance extends to several key areas:
1. Stoichiometry and Chemical Reactions
Molecular mass is essential for performing stoichiometric calculations. Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Knowing the molecular mass allows us to convert between mass and moles of a substance, enabling accurate predictions of reactant amounts and product yields. This is fundamental in many chemical processes, from industrial synthesis to laboratory experiments.
2. Gas Laws and Ideal Gas Behavior
The molecular mass plays a vital role in understanding the behavior of gases. The ideal gas law (PV = nRT) relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). Since the number of moles (n) is directly related to the mass of the gas and its molecular mass, the molecular mass is crucial in predicting and analyzing the behavior of gases like CCl₄ under various conditions.
3. Solution Chemistry and Concentration Calculations
In solution chemistry, molecular mass is critical for calculating the molarity, molality, and other concentration units. Molarity, for example, is defined as moles of solute per liter of solution. Knowing the molecular mass allows us to convert the mass of solute to moles, enabling precise control over solution concentrations. This is vital in analytical chemistry, biochemistry, and many other fields.
4. Physical Properties and Phase Transitions
The molecular mass influences several physical properties of a substance, including its melting point, boiling point, density, and viscosity. The intermolecular forces within a substance, which are related to its molecular mass and structure, significantly impact these properties. Understanding these relationships is crucial in material science and engineering.
5. Spectroscopy and Molecular Identification
Molecular mass can be determined experimentally using various spectroscopic techniques like mass spectrometry. Mass spectrometry provides a precise measurement of the mass-to-charge ratio of ions, allowing for the identification and quantification of molecules like CCl₄ in complex mixtures. This is an indispensable tool in analytical chemistry and environmental monitoring.
6. Environmental Science and Toxicity Studies
Understanding the molecular mass of CCl₄ is critical in environmental science and toxicology. The mass of the molecule influences its behavior in the environment, including its transport, distribution, and persistence. Knowing the molecular mass is vital for modeling its fate in the environment and assessing its potential toxicity and environmental impact. This is crucial for developing appropriate remediation strategies and safety guidelines.
Safety Precautions and Environmental Concerns
While the molecular mass of CCl₄ is a fundamental property, it's essential to acknowledge its toxicity and environmental impact. Carbon tetrachloride is a highly toxic substance that can cause serious health problems, including liver damage, kidney damage, and nervous system disorders. Exposure to CCl₄ should be avoided, and appropriate safety measures should always be taken when handling this chemical. Furthermore, CCl₄ is known to deplete the ozone layer, contributing to climate change. Its use has been significantly restricted due to its harmful effects.
Conclusion: The Significance of Molecular Mass Beyond CCl₄
The molecular mass of CCl₄, calculated as 153.81 amu, is not just a simple numerical value. It's a fundamental property that has far-reaching implications across numerous scientific and industrial disciplines. The concept of molecular mass extends to all molecules, playing a pivotal role in understanding chemical reactions, gas behavior, solution chemistry, physical properties, and environmental interactions. While the case of CCl₄ highlights the importance of molecular mass alongside safety concerns, the broader applications of this concept underscore its significance in the advancement of science and technology. Understanding and accurately calculating molecular mass is essential for accurate scientific research, effective industrial processes, and responsible environmental management.
Latest Posts
Latest Posts
-
A Fatty Acid Is Unsaturated If It
Mar 21, 2025
-
Mass Of A Proton In Grams
Mar 21, 2025
-
Is Glycogen A Monosaccharide Disaccharide Or Polysaccharide
Mar 21, 2025
-
C2h6 O2 Co2 H2o
Mar 21, 2025
-
How Many Total Electrons Does Carbon Have
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Mass Of Ccl4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
