Mass Of A Proton In Grams
News Leon
Mar 21, 2025 · 6 min read
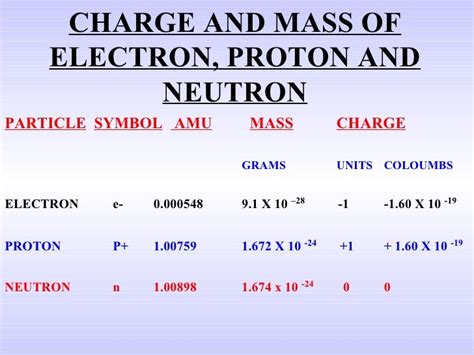
Table of Contents
The Mass of a Proton in Grams: A Deep Dive into Subatomic Physics
The proton. A tiny, fundamental constituent of matter, yet crucial to the very existence of the universe as we know it. Understanding its properties, particularly its mass, is fundamental to comprehending the workings of atoms, nuclei, and the cosmos itself. This article delves deep into the mass of a proton, exploring its measurement, significance, and implications in various fields of physics. We'll unpack the complexities, revealing how this seemingly insignificant number plays a crucial role in our understanding of the universe.
Defining the Proton and its Mass
A proton is a subatomic particle, carrying a single positive electric charge and a mass significantly greater than an electron. It's one of the two main components of an atom's nucleus, alongside the neutron (which carries no charge). The mass of a proton is a fundamental constant in physics, playing a critical role in various calculations and theoretical models. While often expressed in atomic mass units (amu), we’ll focus on its equivalent in grams.
The Challenge of Measuring Subatomic Particles
Measuring the mass of something as infinitesimally small as a proton presents significant challenges. Unlike macroscopic objects that can be readily weighed using scales, subatomic particles require sophisticated techniques and instruments. These techniques often rely on indirect measurements derived from analyzing their interactions with other particles or electromagnetic fields.
Converting Atomic Mass Units (amu) to Grams
The mass of a proton is frequently expressed in atomic mass units (amu), a unit defined relative to the mass of a carbon-12 atom. One amu is approximately 1.66053907 × 10⁻²⁴ grams. To convert the proton's mass from amu to grams, we use this conversion factor. The commonly accepted value for the proton mass is approximately 1.007276466879 amu. Therefore, the mass of a proton in grams is approximately:
1.007276466879 amu * 1.66053907 × 10⁻²⁴ g/amu ≈ 1.6726219 × 10⁻²⁴ grams
This incredibly small number highlights the minuscule scale of the subatomic world.
The Significance of the Proton's Mass
The proton's mass isn't just a mere number; it has profound implications across several branches of physics:
1. Nuclear Physics and Stability
The mass of the proton is pivotal in determining the stability of atomic nuclei. The strong nuclear force, responsible for binding protons and neutrons within the nucleus, must overcome the electromagnetic repulsion between positively charged protons. The proton's mass, coupled with the strong force's strength, dictates which combinations of protons and neutrons form stable nuclei and which undergo radioactive decay. Variations in proton mass would drastically alter the landscape of stable elements and the periodic table itself.
2. Particle Physics and the Standard Model
The proton's mass is an essential parameter within the Standard Model of particle physics, the theoretical framework that describes fundamental particles and their interactions. The Standard Model doesn't directly predict the proton's mass; rather, it's a measured quantity that constrains and tests the model's accuracy. Discrepancies between the measured and predicted mass could indicate physics beyond the Standard Model. Precise measurements of the proton's mass, therefore, are crucial for refining our understanding of fundamental particle interactions.
3. Astrophysics and Cosmology
The mass of the proton is crucial in astrophysical and cosmological contexts. Stars are giant fusion reactors, converting hydrogen (protons) into helium and other heavier elements. The mass-energy equivalence (E=mc²) dictates the immense energy released during these fusion processes, powering stars and shaping the evolution of galaxies. Understanding the proton's mass is vital to accurately modeling stellar evolution and nucleosynthesis, processes that produce the elements that make up planets, stars, and everything in the universe.
4. Chemistry and Atomic Structure
The proton's mass, while small, influences chemical properties. The number of protons in an atom's nucleus defines its atomic number, which in turn determines the element's chemical behavior. Isotopes, atoms of the same element with varying numbers of neutrons, have slightly different masses due to the neutron’s contribution but retain the same chemical properties because the number of protons remains constant.
Measurement Techniques and Uncertainties
Measuring the proton's mass involves complex experimental techniques, primarily relying on mass spectrometry and other particle physics experiments.
Mass Spectrometry:
Mass spectrometry techniques analyze the mass-to-charge ratio of ions. By accurately measuring the ratio and knowing the charge, scientists can deduce the mass of the ion, including protons. Modern mass spectrometers are extremely precise, capable of measuring mass differences with astonishing accuracy.
Particle Physics Experiments:
High-energy particle collisions in experiments such as those conducted at CERN's Large Hadron Collider (LHC) provide indirect measurements of the proton's mass through the analysis of collision products and energy conservation principles. These experiments probe the inner structure of the proton, revealing insights into its constituent quarks and gluons and indirectly contributing to the precision of its mass determination.
Uncertainties and Refinements:
Despite the sophisticated techniques employed, there's always some degree of uncertainty associated with the measured value of the proton's mass. These uncertainties stem from various sources, including systematic errors in experimental setups and limitations in the theoretical models used to analyze the data. Ongoing research constantly refines the measurement, pushing the limits of precision and reducing uncertainties.
Implications for Future Research
The pursuit of ever more precise measurements of the proton's mass is vital for several reasons:
Testing the Standard Model:
A more precise measurement of the proton's mass can provide critical tests of the Standard Model of particle physics. Any significant deviation between the experimental value and theoretical predictions could signal the existence of new physics beyond the Standard Model, opening up exciting avenues of research.
Refining Fundamental Constants:
The proton's mass is related to other fundamental constants in physics, such as the fine-structure constant and the Planck constant. Improvements in the proton's mass measurement contribute to refining our knowledge of these fundamental constants and their interrelationships, improving our overall understanding of the universe's fundamental laws.
Developing New Technologies:
Precise knowledge of the proton's mass is vital in various technological applications, including advanced materials science, medical imaging, and nuclear energy production. Further advancements in measurement techniques will lead to improvements in these technologies.
Conclusion: The Tiny Giant
The mass of a proton, a seemingly insignificant number on its own, holds immense significance in our understanding of the universe. From the stability of atomic nuclei to the energy powering stars and the validation of fundamental physical theories, the proton's mass is a cornerstone of modern physics. Ongoing research, fueled by advancements in measurement techniques and theoretical models, continually refines our knowledge of this fundamental constant, pushing the boundaries of our understanding and paving the way for new discoveries and technological innovations. The journey into the subatomic world reveals a universe of complexity and wonder, and the humble proton, with its precisely defined mass, remains a key player in this unfolding story.
Latest Posts
Latest Posts
-
Which Of The Following Is Monomial
Mar 21, 2025
-
True Or False The Vertical Axis Is Called The Y Axis
Mar 21, 2025
-
Compressing A File Is Also Called
Mar 21, 2025
-
Bundles Of Axons In The Central Nervous System Are Called
Mar 21, 2025
-
How To Find Average Velocity On A Velocity Time Graph
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Mass Of A Proton In Grams . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
