What Is The Bond Order Of No
News Leon
Mar 31, 2025 · 6 min read
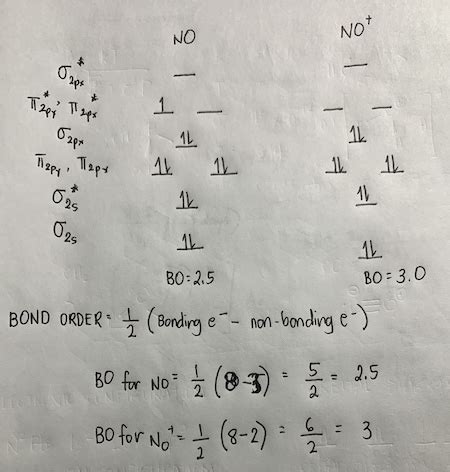
Table of Contents
What is the Bond Order of NO? A Deep Dive into Nitric Oxide's Molecular Structure
Nitric oxide (NO), a simple yet remarkably important molecule, plays a crucial role in various biological and chemical processes. Understanding its properties, particularly its bond order, is key to comprehending its reactivity and function. This article will delve deep into the determination of NO's bond order, exploring different approaches and providing a comprehensive understanding of its molecular structure and bonding.
Understanding Bond Order: A Foundation
Before diving into the specifics of nitric oxide, let's establish a clear understanding of what bond order means. In simple terms, bond order is the number of chemical bonds between a pair of atoms. It's a crucial indicator of the strength and stability of a chemical bond. A higher bond order generally translates to a stronger and shorter bond.
For diatomic molecules like NO, bond order can be calculated using the molecular orbital (MO) theory. This theory describes the behavior of electrons in molecules by considering the combination of atomic orbitals to form molecular orbitals. Electrons occupy these molecular orbitals according to the Aufbau principle and Hund's rule, similar to how they fill atomic orbitals.
Determining the Bond Order of NO using Molecular Orbital Theory
Nitric oxide has a total of 11 valence electrons (5 from nitrogen and 6 from oxygen). To determine its bond order using MO theory, we need to construct the molecular orbital diagram. The diagram will show the energy levels of the molecular orbitals formed by the combination of atomic orbitals from nitrogen and oxygen.
The order of energy levels for diatomic molecules generally follows this pattern (though exceptions exist): σ2s, σ2s, σ2p, π2p, π2p, σ*2p. However, the exact energy ordering can vary depending on the specific atoms involved and can be influenced by the electronegativity difference. In the case of NO, the oxygen 2p orbitals are slightly lower in energy than the nitrogen 2p orbitals.
Building the Molecular Orbital Diagram for NO:
-
Atomic Orbitals: Start by considering the valence atomic orbitals of nitrogen and oxygen: 2s and 2p orbitals for each atom.
-
Molecular Orbital Formation: Combine these atomic orbitals to form molecular orbitals: bonding (σ and π) and antibonding (σ* and π*).
-
Electron Filling: Fill the molecular orbitals with the 11 valence electrons, following the Aufbau principle (lowest energy levels first) and Hund's rule (maximizing unpaired electrons).
-
Bond Order Calculation: The bond order is calculated as half the difference between the number of electrons in bonding orbitals and the number of electrons in antibonding orbitals:
Bond Order = (Number of electrons in bonding orbitals - Number of electrons in antibonding orbitals) / 2
Following this process for NO, the electron configuration will be: σ2s², σ2s², σ2p², π2p⁴, π2p¹.
Therefore:
Bond Order = (8 - 3) / 2 = 2.5
Thus, the bond order of nitric oxide (NO) is 2.5. This indicates a bond stronger than a double bond (bond order 2) but weaker than a triple bond (bond order 3). This fractional bond order is a direct consequence of the presence of unpaired electrons in the antibonding orbital. This unpaired electron contributes to NO's paramagnetism.
The Significance of the 2.5 Bond Order in NO's Properties
The 2.5 bond order of NO is not merely a theoretical number; it has significant implications for its observed properties:
-
Bond Length: The bond length of NO is shorter than a typical double bond but longer than a typical triple bond, consistent with its fractional bond order.
-
Bond Strength: The bond strength of NO reflects its intermediate bond order, being stronger than a double bond but weaker than a triple bond. This intermediate strength influences its reactivity.
-
Paramagnetism: The presence of an unpaired electron in the π*2p antibonding orbital makes NO paramagnetic, meaning it is attracted to a magnetic field. This is a direct consequence of the fractional bond order and the resulting unpaired electron.
-
Reactivity: The relatively high bond order contributes to NO's reactivity. However, the presence of the unpaired electron also makes it a free radical, increasing its reactivity even further. This reactivity underlies its diverse biological and chemical roles.
NO's Role in Biology and Chemistry
Nitric oxide's unique properties, stemming from its 2.5 bond order and resulting unpaired electron, allow it to participate in a wide range of crucial biological and chemical processes:
Biological Roles:
-
Signaling Molecule: NO is a vital signaling molecule in various biological systems. It plays a role in vasodilation (widening of blood vessels), neurotransmission (transmission of nerve impulses), and immune response.
-
Enzyme Activity: NO interacts with various enzymes, modulating their activity and influencing cellular processes.
-
Defense Mechanism: In some organisms, NO acts as a defense mechanism against pathogens.
Chemical Roles:
-
Industrial Processes: NO is involved in several industrial processes, including the production of nitric acid.
-
Catalysis: NO can act as a catalyst in certain chemical reactions.
-
Environmental Chemistry: NO is a significant component of air pollution and plays a role in the formation of acid rain and smog. Understanding its reactivity is crucial for developing strategies to mitigate its environmental impact.
Further Considerations and Advanced Topics
While the basic MO theory provides a good understanding of NO's bond order, more sophisticated methods can provide a more accurate and nuanced picture. These include:
-
Density Functional Theory (DFT): DFT is a powerful computational method that can accurately predict the electronic structure and properties of molecules, offering a refined approach to calculating bond order.
-
Post-Hartree-Fock methods: These advanced quantum chemical methods provide even more accurate calculations of molecular properties, including bond order, although they are computationally more demanding.
The discussion of NO's bond order is not solely confined to the simple application of molecular orbital theory. It necessitates a comprehensive understanding of its electronic structure, which provides a foundation for explaining the molecule's reactivity and its significant impact across various scientific disciplines. Investigating these advanced methods offers a more precise understanding of the subtleties of its bonding and behaviour.
Conclusion
The bond order of nitric oxide (NO) is 2.5, a direct result of its molecular orbital configuration. This fractional bond order is not an anomaly but a critical feature that dictates its unique properties and the significant role it plays in biological systems and chemical processes. Understanding the 2.5 bond order of NO is key to appreciating its reactivity, paramagnetism, and multifaceted importance in diverse fields. By applying MO theory and potentially more advanced computational methods, we can gain a deeper and more comprehensive appreciation for this fascinating molecule and its profound impact on our world.
Latest Posts
Latest Posts
-
All Of The Following Refer To Mitosis Except
Apr 01, 2025
-
Which Of The Following Temperatures Is The Coldest
Apr 01, 2025
-
A Short Term Unsecured Promissory Note Issued By A Company Is
Apr 01, 2025
-
Adjacent Angles Whose Sum In 180 Degrees
Apr 01, 2025
-
Lewis Dot Structure For Magnesium Chloride
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Bond Order Of No . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
