What Are The Three Parts Of An Atp Molecule
News Leon
Mar 16, 2025 · 6 min read
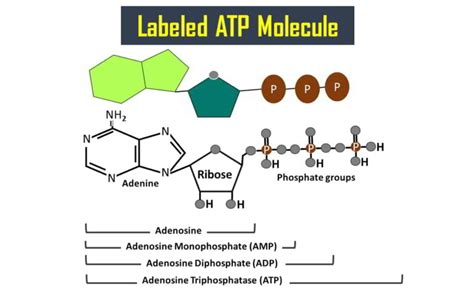
Table of Contents
What are the Three Parts of an ATP Molecule? Unlocking the Energy Currency of Life
Adenosine triphosphate (ATP) is the primary energy currency of all living cells. It's the molecule that powers virtually every biological process, from muscle contraction and nerve impulse transmission to protein synthesis and DNA replication. Understanding the structure of ATP is crucial to understanding how it functions as this vital energy carrier. This article will delve deep into the three fundamental components of an ATP molecule, exploring their individual roles and how they contribute to ATP's overall energy-carrying capacity.
The Tripartite Structure of ATP: Adenine, Ribose, and Phosphate Groups
The ATP molecule is remarkably compact yet incredibly powerful. Its structure is deceptively simple, consisting of three key components:
- Adenine: A nitrogenous base, a fundamental building block of nucleic acids like DNA and RNA.
- Ribose: A five-carbon sugar, providing the structural backbone of the molecule.
- Triphosphate group: A chain of three phosphate groups linked together, the source of ATP's readily available energy.
Let's explore each of these components in more detail.
1. Adenine: The Nitrogenous Base
Adenine (A) is a purine base, meaning it's a double-ringed structure containing nitrogen atoms. It's one of the four nitrogenous bases found in DNA and RNA (along with guanine, cytosine, and thymine/uracil). In ATP, adenine is attached to the ribose sugar through a glycosidic bond, specifically at the 1' carbon atom of the ribose. This nitrogenous base plays a crucial role in the recognition and binding of ATP to enzymes and other proteins involved in ATP metabolism. The specific arrangement of atoms within adenine contributes to its ability to participate in hydrogen bonding, an essential interaction for ATP's function within cellular processes. The unique chemical properties of adenine influence the overall stability and reactivity of the ATP molecule.
2. Ribose: The Five-Carbon Sugar Backbone
Ribose is a pentose sugar, meaning it contains five carbon atoms. In ATP, it's specifically β-D-ribose, a crucial component that dictates the molecule's overall three-dimensional structure. The ribose sugar acts as a bridge, connecting the adenine base to the triphosphate group. The five carbon atoms of ribose are numbered 1' to 5', and the adenine is attached at the 1' carbon. The triphosphate group is attached to the 5' carbon atom of the ribose. This arrangement is vital for the molecule's stability and the accessibility of the phosphate groups for energy transfer. The hydroxyl groups on the ribose sugar also contribute to the molecule's overall polarity and solubility in water, allowing it to move freely within the aqueous environment of the cell.
3. The Triphosphate Group: The Energy Powerhouse
The triphosphate group is the most crucial component of the ATP molecule, being the source of its readily available energy. This group consists of three phosphate molecules (Pi) linked together by two high-energy phosphoanhydride bonds. These bonds are characterized by a significant amount of potential energy stored within them. The energy is stored in these bonds because the negatively charged phosphate groups repel each other. This repulsion creates an unstable, high-energy configuration. This instability makes the phosphate bonds relatively easy to break, and it is the breaking of these bonds that releases the stored energy to drive various cellular processes.
Hydrolysis, a chemical reaction where water is added to break a bond, is the key process involved in energy release from ATP. When one phosphate group is cleaved off, the molecule becomes adenosine diphosphate (ADP), releasing a considerable amount of energy in the process. The same principle applies to the further hydrolysis of ADP to adenosine monophosphate (AMP), with the release of additional energy.
The High-Energy Phosphoanhydride Bonds: A Closer Look
The high-energy phosphoanhydride bonds are what truly distinguishes ATP as the cell's energy currency. These bonds are not just "high-energy" in a relative sense; they possess a significantly larger amount of free energy compared to other types of chemical bonds found in biological molecules. This high-energy content stems from several factors:
- Electrostatic repulsion: The negatively charged phosphate groups strongly repel each other, making the bond inherently unstable.
- Resonance stabilization: The products of ATP hydrolysis (ADP and inorganic phosphate) are more resonance-stabilized than ATP itself, meaning they are more stable and have lower energy.
- Increased hydration: The products of hydrolysis are more effectively hydrated (surrounded by water molecules) than ATP. This increased hydration further stabilizes the products and releases energy.
These factors combine to ensure that the hydrolysis of ATP is an exergonic reaction – a reaction that releases energy. This released energy can then be coupled to other endergonic reactions (reactions that require energy input) within the cell, driving them forward. This coupling of exergonic and endergonic reactions is a fundamental principle of cellular energy metabolism.
ATP's Role in Cellular Processes: A Versatile Energy Carrier
ATP's significance lies not only in its structure but also in its remarkable versatility as an energy carrier. It serves as the intermediary between energy-releasing processes (like cellular respiration) and energy-requiring processes (like muscle contraction or biosynthesis). Here are some key examples of ATP's involvement in cellular functions:
- Muscle contraction: ATP provides the energy for the interaction between actin and myosin filaments, leading to muscle contraction.
- Active transport: ATP powers membrane pumps that move ions and molecules against their concentration gradients, maintaining cellular homeostasis.
- Nerve impulse transmission: The transmission of nerve impulses relies on ATP-driven ion pumps to maintain the electrochemical gradients across neuronal membranes.
- Protein synthesis: ATP is required for the formation of peptide bonds during protein synthesis, driving the energy-consuming process of translation.
- DNA replication: The unwinding of the DNA double helix and the subsequent synthesis of new DNA strands are ATP-dependent processes.
- Cell signaling: ATP acts as a signaling molecule in some cellular processes, triggering downstream effects.
ATP Regeneration: The Continuous Energy Cycle
ATP is not a static molecule; it's constantly being used and regenerated within the cell. The continuous cycle of ATP hydrolysis and regeneration ensures a constant supply of energy to power cellular activities. The main pathway for ATP regeneration is cellular respiration, a series of metabolic reactions that break down glucose and other fuels to generate ATP. Other processes, such as fermentation, can also contribute to ATP regeneration, albeit with lower efficiency. This continuous cycle of ATP hydrolysis and synthesis is a testament to the dynamic nature of energy metabolism in living cells.
Conclusion: ATP, The Master Molecule of Life
In conclusion, the three parts of an ATP molecule – adenine, ribose, and the triphosphate group – work together in a beautifully orchestrated arrangement to create the essential energy currency of life. Understanding the specific contributions of each component, particularly the high-energy phosphoanhydride bonds, sheds light on ATP's ability to efficiently store and release energy to fuel the myriad of life's processes. The continuous cycle of ATP synthesis and hydrolysis highlights the dynamic and crucial role ATP plays in maintaining cellular function and powering the remarkable complexity of living organisms. Its significance in all forms of life underlines its importance as a fundamental molecule central to understanding biological energy and life itself. Further research into ATP's intricate interactions with cellular machinery continues to unlock even more of its secrets, continually reinforcing its position as the ultimate energy powerhouse of the cell.
Latest Posts
Latest Posts
-
What Part Of The Ear Looks Like A Snail Shell
Mar 17, 2025
-
What Is The Molar Mass Of Agno3
Mar 17, 2025
-
What Percent Of 68 Is 17
Mar 17, 2025
-
Which Bond Is The Most Polar
Mar 17, 2025
-
Burning Of Candle Is Chemical Change
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Are The Three Parts Of An Atp Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
