What 3 Things Make Up A Nucleotide
News Leon
Mar 22, 2025 · 6 min read
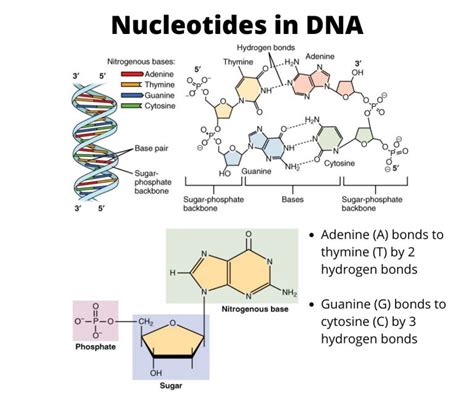
Table of Contents
What 3 Things Make Up a Nucleotide? A Deep Dive into the Building Blocks of Life
Nucleotides: the very foundation of our genetic code, the driving force behind cellular energy, and key players in countless cellular processes. Understanding their composition is crucial to grasping the complexities of life itself. This comprehensive guide will delve into the three essential components that make up a nucleotide, exploring their individual characteristics and their collective significance in the grand scheme of biology.
The Triad of Nucleotide Composition: Sugar, Base, and Phosphate
Every nucleotide, regardless of its specific role in the body, consists of three fundamental components:
- A Pentose Sugar: A five-carbon sugar molecule.
- A Nitrogenous Base: A ringed structure containing nitrogen atoms.
- A Phosphate Group: A phosphorus atom bonded to four oxygen atoms.
Let's explore each component in detail:
1. The Pentose Sugar: The Backbone of the Nucleotide
The pentose sugar acts as the central structural element of a nucleotide, providing the framework to which the other two components attach. There are two primary types of pentose sugars found in nucleotides:
a) Ribose:
Ribose is a five-carbon sugar with a hydroxyl (-OH) group attached to the 2' carbon atom. This hydroxyl group plays a critical role in the reactivity and overall chemical properties of ribonucleotides, the building blocks of RNA (ribonucleic acid). The presence of this hydroxyl group makes RNA less stable than DNA. RNA is more susceptible to hydrolysis, a chemical reaction where water breaks down the molecule. This inherent instability is partly why RNA is primarily involved in transient processes, like protein synthesis, whereas DNA serves as the long-term repository of genetic information.
b) Deoxyribose:
Deoxyribose is structurally similar to ribose, but it lacks a hydroxyl group at the 2' carbon atom. Instead, it has a hydrogen atom (-H) at that position. This seemingly small difference has profound implications for the stability and structure of DNA (deoxyribonucleic acid). The absence of the 2'-hydroxyl group makes DNA significantly more stable and less susceptible to hydrolysis compared to RNA. This increased stability is essential for the long-term storage of genetic information, a key function of DNA. The double helix structure of DNA further enhances this stability.
2. The Nitrogenous Base: The Information Carrier
The nitrogenous base is the information-carrying component of a nucleotide. It is responsible for the diversity and specificity of nucleic acids, forming the genetic code that dictates the characteristics of an organism. These bases are categorized into two main groups:
a) Purines:
Purines are double-ringed structures consisting of a six-membered ring fused to a five-membered ring. There are two principal purines found in nucleotides:
-
Adenine (A): Adenine is a crucial component of both DNA and RNA. It pairs with thymine (in DNA) or uracil (in RNA) through hydrogen bonds, forming the fundamental building blocks of the genetic code. Adenine also plays a vital role in energy transfer, as adenosine triphosphate (ATP) is the primary energy currency of cells.
-
Guanine (G): Guanine, like adenine, is found in both DNA and RNA. It pairs with cytosine through hydrogen bonds, contributing to the structure and stability of the double helix in DNA and the secondary structure in RNA.
b) Pyrimidines:
Pyrimidines are single-ringed structures. The primary pyrimidines in nucleotides are:
-
Cytosine (C): Cytosine is found in both DNA and RNA, pairing with guanine through hydrogen bonds. It plays a crucial role in the genetic code and is a vital component of the structural integrity of nucleic acids.
-
Thymine (T): Thymine is found exclusively in DNA, pairing with adenine through hydrogen bonds. Its presence in DNA contributes significantly to the stability of the double helix structure.
-
Uracil (U): Uracil is found exclusively in RNA, pairing with adenine through hydrogen bonds. It replaces thymine in RNA and plays a critical role in the processes of transcription and translation.
The specific sequence of these nitrogenous bases along the sugar-phosphate backbone dictates the genetic information encoded within DNA and RNA. This sequence determines the amino acid sequence of proteins and ultimately the phenotype of an organism.
3. The Phosphate Group: Linking Nucleotides and Providing Energy
The phosphate group is the negatively charged component of a nucleotide. It is composed of a phosphorus atom covalently bonded to four oxygen atoms, giving it a crucial role in several key functions:
-
Linking Nucleotides: The phosphate group forms the phosphodiester bond, connecting the 3' carbon of one sugar molecule to the 5' carbon of the next sugar molecule. This linkage creates the sugar-phosphate backbone of DNA and RNA, the continuous chain upon which the nitrogenous bases are attached. The directionality (5' to 3') of this backbone is crucial for the replication and transcription processes.
-
Energy Transfer: The phosphate groups in nucleotides like ATP (adenosine triphosphate) store considerable chemical energy. The hydrolysis of these phosphate bonds, releasing energy, fuels countless cellular processes, making ATP the primary energy currency of the cell. Other nucleotides like GTP (guanosine triphosphate) and UTP (uridine triphosphate) also participate in energy transfer reactions within the cell.
-
Cellular Signaling: Nucleotides and their derivatives are involved in a wide range of cellular signaling pathways. Cyclic AMP (cAMP), a derivative of ATP, is a crucial second messenger in many hormonal signaling cascades. These signaling pathways are crucial for regulating diverse cellular processes, including cell growth, differentiation, and metabolism.
-
Enzyme Cofactors: Some nucleotides act as coenzymes, essential components of many enzymatic reactions. For example, NAD+ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide) are critical electron carriers in metabolic pathways such as respiration and photosynthesis. These coenzymes assist enzymes in carrying out their catalytic functions, enabling vital biological processes to occur.
Nucleotide Variations and Their Functions
The combination of different pentose sugars, nitrogenous bases, and the number of phosphate groups leads to a wide variety of nucleotides, each with specialized functions:
-
Mononucleotides: These consist of a single nucleotide unit, like ATP, GTP, cAMP, etc. They often play crucial roles in energy transfer and cellular signaling.
-
Dinucleotides: Two nucleotides linked together, such as FAD and NAD+. These commonly function as coenzymes in metabolic reactions.
-
Polynucleotides: Long chains of nucleotides linked together by phosphodiester bonds, forming DNA and RNA. These are the carriers of genetic information and play essential roles in gene expression.
Conclusion: The Significance of Understanding Nucleotide Structure
The three components of a nucleotide – pentose sugar, nitrogenous base, and phosphate group – are intricately interwoven to form the building blocks of life. Understanding their individual characteristics and their interactions is critical to comprehending fundamental biological processes, from the transmission of genetic information to the generation and utilization of cellular energy. The diverse roles of nucleotides extend far beyond these basics, highlighting their importance in maintaining cellular homeostasis and enabling the complexity of life as we know it. The precise arrangement of these three components allows for the enormous diversity and complexity of biological systems, emphasizing their central role in the molecular machinery of life. Further research continues to unveil the intricacies and significance of these fundamental molecules, constantly expanding our understanding of the biological world.
Latest Posts
Latest Posts
-
What Are Two Subatomic Particles Found In The Nucleus
Mar 22, 2025
-
What Is The Monomer Of Cellulose
Mar 22, 2025
-
Which Of The Following Sequence Is Correct
Mar 22, 2025
-
No Mans Sky 1 2 6 24 120
Mar 22, 2025
-
Number Of Electrons In A 2p Orbital
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What 3 Things Make Up A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
