Number Of Electrons In A 2p Orbital
News Leon
Mar 22, 2025 · 5 min read
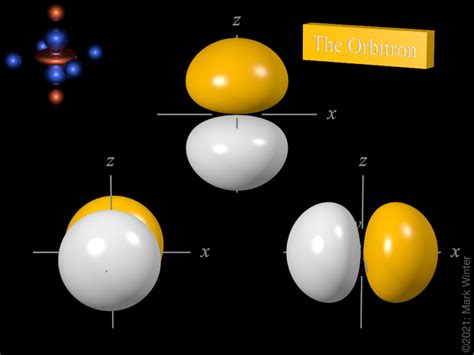
Table of Contents
Delving Deep: Understanding the Number of Electrons in a 2p Orbital
The seemingly simple question, "How many electrons can a 2p orbital hold?" opens the door to a fascinating exploration of atomic structure, quantum mechanics, and the very nature of electron behavior within an atom. While the answer itself is straightforward, understanding the why behind it requires a deeper dive into the fundamental principles governing the arrangement of electrons. This article will comprehensively explore this topic, covering key concepts like electron shells, subshells, orbitals, and the Pauli Exclusion Principle, ultimately clarifying the electron capacity of a 2p orbital and its implications.
Understanding Atomic Structure: Shells, Subshells, and Orbitals
Before we tackle the 2p orbital specifically, let's establish a foundational understanding of atomic structure. Atoms are composed of a nucleus containing protons and neutrons, surrounded by a cloud of electrons. These electrons are not randomly distributed; they occupy specific energy levels, or shells, arranged in increasing distance from the nucleus. Each shell can hold a maximum number of electrons, determined by the formula 2n², where 'n' represents the principal quantum number (shell number).
Within each shell, electrons are further organized into subshells, denoted by the letters s, p, d, and f. These subshells represent different energy levels within a shell. The number of subshells in a shell is equal to the shell's principal quantum number (n). For instance, the first shell (n=1) only has one subshell (s), the second shell (n=2) has two subshells (s and p), and so on.
Finally, within each subshell are orbitals. Orbitals are regions of space where there is a high probability of finding an electron. Each subshell contains a specific number of orbitals:
- s subshell: 1 orbital
- p subshell: 3 orbitals
- d subshell: 5 orbitals
- f subshell: 7 orbitals
This hierarchical organization—shells, subshells, and orbitals—is crucial for understanding electron configuration and the maximum number of electrons each orbital can accommodate.
The 2p Subshell: A Closer Look
Now, let's focus on the 2p subshell. As mentioned earlier, the principal quantum number (n) for the second shell is 2. Therefore, the second shell contains two subshells: 2s and 2p. The 2p subshell, with its three orbitals, is where things get interesting regarding electron capacity.
Each of the three 2p orbitals is designated with a specific magnetic quantum number (ml), which can have values of -1, 0, and +1. These orbitals are often depicted as dumbbell-shaped regions of space oriented along the x, y, and z axes. While these shapes are helpful visualizations, it's crucial to remember that they represent regions of probability, not precise electron locations.
The Pauli Exclusion Principle: A Limiting Factor
The key to determining the maximum number of electrons a 2p orbital can hold lies in the Pauli Exclusion Principle. This fundamental principle of quantum mechanics states that no two electrons in an atom can have the same set of four quantum numbers. These four quantum numbers are:
- Principal quantum number (n): Defines the electron shell.
- Azimuthal quantum number (l): Defines the subshell (s, p, d, f).
- Magnetic quantum number (ml): Defines the specific orbital within a subshell.
- Spin quantum number (ms): Describes the intrinsic angular momentum of the electron, with values of +1/2 (spin up) or -1/2 (spin down).
Because each electron must have a unique set of four quantum numbers, each orbital—regardless of its type (s, p, d, f)—can hold a maximum of two electrons. These two electrons must have opposite spins (+1/2 and -1/2).
Calculating the Maximum Number of Electrons in the 2p Subshell
With this understanding, we can now calculate the maximum number of electrons the 2p subshell can hold.
- The 2p subshell has three orbitals.
- Each orbital can hold a maximum of two electrons (due to the Pauli Exclusion Principle).
- Therefore, the 2p subshell can hold a maximum of 3 orbitals * 2 electrons/orbital = 6 electrons.
Implications and Further Exploration
The capacity of the 2p subshell to hold six electrons has significant implications for the chemical behavior of elements. The filling of the 2p subshell influences the valence electrons, which are the outermost electrons involved in chemical bonding. Elements with partially filled 2p subshells often exhibit diverse and interesting chemical properties.
The arrangement of electrons within the 2p subshell and other subshells also dictates the reactivity of elements. Elements strive to achieve a stable electron configuration, often by gaining, losing, or sharing electrons to fill their outer shells. Understanding the electron distribution in atoms is fundamental to comprehending the periodic table's structure and the trends in elemental properties.
Furthermore, exploring the concept of electron orbitals leads to a deeper appreciation of quantum mechanics. The probabilistic nature of electron location and the wave-particle duality of electrons are central concepts in modern physics.
Beyond the 2p Orbital: A Broader Perspective
While this article has focused on the 2p orbital, the principles discussed apply broadly to all orbitals and subshells. Understanding the electron capacity of other orbitals (e.g., 3d, 4f) is crucial for comprehending the electron configurations of heavier elements and their unique properties. The arrangement of electrons, influenced by the Pauli Exclusion Principle and Hund's Rule (which states that electrons will individually occupy each orbital within a subshell before doubling up), determines the chemical and physical behavior of all matter.
Conclusion: The Significance of Understanding Electron Configuration
The seemingly simple question of how many electrons a 2p orbital can hold leads to a rich understanding of atomic structure, quantum mechanics, and the fundamental principles governing the behavior of matter. The capacity of six electrons, governed by the Pauli Exclusion Principle, is not merely a numerical fact; it’s a cornerstone of our understanding of chemical bonding, reactivity, and the periodic table. This foundational knowledge is crucial for anyone pursuing studies in chemistry, physics, or related fields. The exploration of electron configuration provides an entry point into the fascinating world of quantum mechanics and its profound implications for our understanding of the universe.
Latest Posts
Latest Posts
-
A Woman Has Twice As Many Dimes As Quarters
Mar 22, 2025
-
How Far Is The Sun Light Years
Mar 22, 2025
-
Concept Map Mechanisms Of Hormone Action
Mar 22, 2025
-
Every Result Has Both Needs Met And Page Quality Sliders
Mar 22, 2025
-
Which Of The Following Is Not A Monomer
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Number Of Electrons In A 2p Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
