What Is The Monomer Of Cellulose
News Leon
Mar 22, 2025 · 5 min read
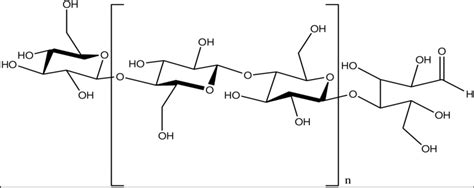
Table of Contents
What is the Monomer of Cellulose? A Deep Dive into the Structure and Function of Nature's Most Abundant Polymer
Cellulose, the most abundant organic polymer on Earth, forms the structural foundation of plant cell walls. Understanding its structure is crucial to appreciating its diverse roles in nature and its potential applications in various industries. This comprehensive guide delves into the fundamental building block of cellulose: its monomer, glucose. We will explore glucose's properties, the process of cellulose synthesis, the unique structural characteristics of cellulose, and its significance in different fields.
Understanding Glucose: The Basic Building Block
Before we delve into the intricacies of cellulose, let's establish a firm understanding of its monomer: glucose. Glucose, a simple sugar, is a hexose monosaccharide, meaning it's a six-carbon sugar molecule. Its chemical formula is C₆H₁₂O₆, and it exists in two primary forms: α-glucose and β-glucose.
α-glucose vs. β-glucose: A Crucial Distinction
The difference between α-glucose and β-glucose lies in the orientation of the hydroxyl (-OH) group on carbon atom 1 (C1). In α-glucose, the hydroxyl group points downwards, while in β-glucose, it points upwards. This seemingly minor difference has profound implications for the properties and function of the resulting polymers.
α-glucose is the monomer of starch and glycogen, both energy storage polymers. The linkage between α-glucose molecules allows for a helical or branched structure, making these polymers readily digestible by enzymes.
β-glucose, on the other hand, is the monomer of cellulose. The linkage between β-glucose molecules results in a linear, rigid structure, making cellulose highly resistant to enzymatic degradation. This difference is key to cellulose's structural role in plants.
The Synthesis of Cellulose: From Glucose to a Robust Polymer
The synthesis of cellulose is a complex process that occurs within the plant cell walls. Specialized enzymes, called cellulose synthases, are responsible for catalyzing the polymerization of β-glucose molecules. These enzymes are located in the plasma membrane and work in coordination with other proteins to facilitate the assembly and secretion of cellulose microfibrils.
The Role of Cellulose Synthases
Cellulose synthases are transmembrane proteins that use UDP-glucose (uridine diphosphate glucose) as a substrate. They sequentially add β-glucose molecules to the growing cellulose chain, forming a β-1,4-glycosidic bond between the C1 carbon of one glucose molecule and the C4 carbon of the next. This specific type of linkage is crucial to cellulose's unique properties.
Cellulose Microfibril Formation: A Hierarchical Structure
The newly synthesized cellulose chains don't exist as isolated molecules. Instead, they aggregate into highly organized structures called cellulose microfibrils. These microfibrils consist of dozens of cellulose chains bundled together through hydrogen bonding, creating a remarkably strong and rigid structure.
The strength and rigidity of cellulose microfibrils are primarily due to the extensive hydrogen bonding between the hydroxyl groups of adjacent glucose units within a single chain and between adjacent chains within the microfibril. This intricate network of hydrogen bonds contributes significantly to the overall strength and stability of the plant cell wall.
The Unique Structure and Properties of Cellulose
The linear arrangement of β-glucose units and the extensive hydrogen bonding between chains are responsible for the remarkable properties of cellulose:
- High Tensile Strength: Cellulose fibers are exceptionally strong, capable of withstanding considerable tensile stress. This property is essential for providing structural support to plants.
- Insolubility in Water: The extensive hydrogen bonding and crystalline structure of cellulose make it insoluble in water, ensuring the stability of plant cell walls under various environmental conditions.
- Crystalline Structure: Cellulose chains are arranged in a highly ordered crystalline structure, contributing to its rigidity and strength. However, some amorphous regions also exist within the cellulose structure.
- Biodegradability (Slow): While cellulose is resistant to degradation by most enzymes, it is eventually biodegradable by specialized microorganisms that produce cellulases. These enzymes can break the β-1,4-glycosidic bonds, releasing glucose molecules.
The Importance of Cellulose: Applications and Significance
Cellulose's abundance and unique properties have led to its widespread use in various industries and its vital role in the environment:
Industrial Applications
- Paper Production: Cellulose is the primary component of paper. Wood pulp, a source of cellulose, is processed to create paper of varying qualities.
- Textiles: Cellulose is the major component of cotton, linen, and other natural fibers, making it crucial for the textile industry.
- Biofuels: Cellulose is a promising source of biofuel, as it can be converted into ethanol through enzymatic hydrolysis and fermentation. Research is ongoing to improve the efficiency of this process.
- Plastics: Cellulose-based plastics are being developed as sustainable alternatives to petroleum-based plastics, offering a more environmentally friendly option.
- Food Industry: Cellulose is used as a food additive, serving as a thickener, stabilizer, and emulsifier in various food products.
Ecological Significance
- Plant Structure: Cellulose forms the main structural component of plant cell walls, providing support and protection for plants.
- Carbon Cycle: Cellulose plays a crucial role in the global carbon cycle. Plants sequester atmospheric carbon dioxide through photosynthesis, incorporating it into cellulose.
- Soil Formation: The decomposition of cellulose contributes to soil formation, releasing nutrients and improving soil structure.
Challenges and Future Research
Despite the extensive use and importance of cellulose, some challenges remain:
- Efficient Cellulose Degradation: The recalcitrance of cellulose to enzymatic degradation remains a challenge for biofuel production. Research is focused on developing more efficient and cost-effective cellulases.
- Sustainable Cellulose Production: Sustainable methods for producing cellulose from renewable sources are crucial to minimize environmental impact.
- New Applications: Research continues to explore new applications for cellulose, including advanced materials, drug delivery systems, and tissue engineering.
Conclusion: The Significance of a Simple Sugar
The seemingly simple monomer, β-glucose, gives rise to the extraordinarily complex and vital polymer, cellulose. Understanding the structure and properties of cellulose, from the level of its individual glucose monomers to its hierarchical organization into microfibrils, is key to appreciating its fundamental role in the natural world and its potential for future applications. Ongoing research into cellulose continues to unlock its potential, paving the way for new technologies and a more sustainable future. From the strength of a tree trunk to the softness of cotton, the impact of this ubiquitous polymer is undeniable. The future of cellulose promises even greater innovation and integration into various aspects of our lives.
Latest Posts
Latest Posts
-
What Is Half Of A 1 2 Teaspoon
Mar 22, 2025
-
1 1 Sin 1 1 Sin
Mar 22, 2025
-
Oxidation Number For Cr In Cr2o72
Mar 22, 2025
-
Which Of The Following Statements About Surfactants Is Not True
Mar 22, 2025
-
How Long Was Rip Van Winkle Asleep
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is The Monomer Of Cellulose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
