Oxidation Number For Cr In Cr2o72
News Leon
Mar 22, 2025 · 5 min read
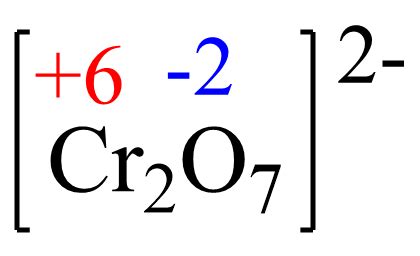
Table of Contents
Determining the Oxidation Number of Cr in Cr₂O₇²⁻: A Comprehensive Guide
The dichromate ion, Cr₂O₇²⁻, is a powerful oxidizing agent frequently encountered in chemistry, particularly in redox reactions. Understanding the oxidation number of chromium (Cr) within this ion is crucial for balancing redox equations and predicting the reactivity of the dichromate ion. This comprehensive guide will delve into the process of determining the oxidation number of Cr in Cr₂O₇²⁻, exploring the underlying principles and offering practical examples.
Understanding Oxidation Numbers
Before we tackle the specific case of Cr₂O₇²⁻, let's establish a solid understanding of oxidation numbers themselves. The oxidation number, also known as oxidation state, is a number assigned to an atom in a chemical compound that represents the hypothetical charge the atom would have if all bonds to atoms of different elements were 100% ionic. This is a crucial concept in understanding electron transfer and redox reactions.
Several rules govern the assignment of oxidation numbers:
-
Rule 1: The oxidation number of an element in its free (uncombined) state is always zero. For example, the oxidation number of O₂ is 0, and the oxidation number of Na is 0.
-
Rule 2: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
Rule 3: The oxidation number of hydrogen is usually +1, except in metal hydrides where it is -1. Examples include HCl (+1) and NaH (-1).
-
Rule 4: The oxidation number of oxygen is usually -2, except in peroxides (like H₂O₂) where it is -1, and in superoxides where it is -1/2.
-
Rule 5: The sum of oxidation numbers of all atoms in a neutral molecule is zero.
-
Rule 6: The sum of oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
Determining the Oxidation Number of Cr in Cr₂O₇²⁻
Now, let's apply these rules to determine the oxidation number of chromium in the dichromate ion, Cr₂O₇²⁻. We know the following:
- The oxidation number of oxygen is typically -2 (Rule 4).
- The overall charge of the dichromate ion is -2 (Rule 6).
Let's denote the oxidation number of chromium as 'x'. Since there are two chromium atoms in the ion, the total contribution of chromium to the overall charge is 2x. There are seven oxygen atoms, each contributing -2 to the charge, resulting in a total contribution of 7(-2) = -14.
According to Rule 6, the sum of oxidation numbers must equal the overall charge of the ion:
2x + 7(-2) = -2
Now, we can solve for x:
2x - 14 = -2 2x = 12 x = +6
Therefore, the oxidation number of chromium (Cr) in the dichromate ion (Cr₂O₇²⁻) is +6.
Significance of the +6 Oxidation State of Chromium
The +6 oxidation state of chromium in Cr₂O₇²⁻ is highly significant for several reasons:
-
Oxidizing Power: Chromium in the +6 oxidation state is a strong oxidizing agent. It readily accepts electrons, undergoing reduction to lower oxidation states (e.g., +3). This property makes dichromate a vital reagent in various oxidation reactions in organic and inorganic chemistry.
-
Color: The intense orange-red color of dichromate solutions is a characteristic feature of chromium in the +6 oxidation state. This color is due to the electronic transitions within the Cr⁶⁺ ion.
-
Applications: The oxidizing power of Cr₂O₇²⁻ finds numerous applications, including:
- Organic Chemistry: Oxidation of alcohols to aldehydes or ketones, oxidation of alkenes.
- Analytical Chemistry: Titrations (redox titrations), detection of reducing agents.
- Industrial Processes: Chrome plating, leather tanning.
Redox Reactions Involving Dichromate
To illustrate the role of the +6 oxidation state of chromium in redox reactions, let's consider a simple example: the reaction of dichromate with iron(II) ions:
Cr₂O₇²⁻(aq) + 6Fe²⁺(aq) + 14H⁺(aq) → 2Cr³⁺(aq) + 6Fe³⁺(aq) + 7H₂O(l)
In this reaction, the dichromate ion (Cr₂O₇²⁻) acts as an oxidizing agent, oxidizing Fe²⁺ to Fe³⁺. Simultaneously, chromium is reduced from the +6 oxidation state in Cr₂O₇²⁻ to the +3 oxidation state in Cr³⁺. Notice the change in oxidation numbers: chromium goes from +6 to +3 (a reduction of 3 electrons per chromium atom), and iron goes from +2 to +3 (an oxidation of 1 electron per iron atom). The equation is balanced by ensuring that the number of electrons lost by iron equals the number of electrons gained by chromium.
Other Chromium Oxidation States
It's important to note that chromium exhibits a range of oxidation states, including +2, +3, +4, +5, and +6. The stability of each oxidation state depends on various factors, such as the pH, the presence of ligands, and the oxidizing or reducing environment. The +3 oxidation state is particularly stable and is the most common oxidation state for chromium in its compounds.
Comparing Chromium Oxidation States:
| Oxidation State | Color (Typical) | Example Compound | Oxidizing/Reducing Power | Stability |
|---|---|---|---|---|
| +2 | Blue | CrCl₂ | Reducing | Relatively unstable |
| +3 | Green/Violet | CrCl₃, Cr₂O₃ | Neither strongly oxidizing nor reducing | Relatively stable |
| +6 | Orange/Red | Cr₂O₇²⁻, CrO₄²⁻ | Oxidizing | Moderately stable in acidic solutions |
Further Exploration and Applications
The determination of oxidation numbers is a fundamental skill in chemistry. Mastering this concept opens doors to understanding a wide array of chemical reactions, including redox reactions, which are crucial in various fields. Further exploration could involve:
- Balancing redox equations: Practice balancing more complex redox equations involving dichromate.
- Electrochemistry: Understanding the role of oxidation numbers in electrochemical cells and electrode potentials.
- Qualitative analysis: Using redox reactions involving dichromate for the identification of specific ions or compounds.
- Industrial processes: Investigating the industrial applications of chromium compounds in different oxidation states.
The dichromate ion (Cr₂O₇²⁻) and its chemistry, particularly the +6 oxidation state of chromium, represent a significant area of study with far-reaching applications across numerous branches of chemistry and beyond. A thorough grasp of the oxidation states and redox reactions involving chromium compounds is essential for any aspiring chemist or anyone interested in the fascinating world of chemical transformations. This detailed explanation provides a comprehensive foundation for further exploration and deeper understanding of this important topic.
Latest Posts
Latest Posts
-
Which Of The Following Is Are Voluntary Muscle
Mar 23, 2025
-
Which Of The Following Is A Property Of Bases
Mar 23, 2025
-
Joule Second Is The Unit Of
Mar 23, 2025
-
All The Plants In A Particular Area
Mar 23, 2025
-
Select The Correct Statement Regarding Epithelia
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number For Cr In Cr2o72 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
