Which Of The Following Is A Property Of Bases
News Leon
Mar 23, 2025 · 7 min read
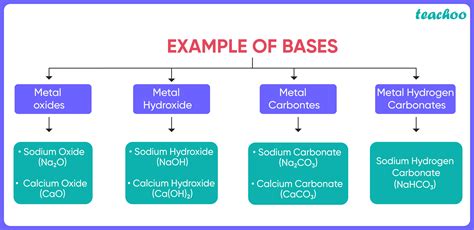
Table of Contents
Which of the Following is a Property of Bases? A Deep Dive into Base Chemistry
Understanding the properties of bases is fundamental to chemistry. Bases, along with acids, form the cornerstone of acid-base chemistry, a crucial concept in numerous scientific fields. This comprehensive guide will explore the key properties that define bases, contrasting them with acids and delving into the intricacies of their behavior. We'll examine several characteristics, clarifying which are indeed properties of bases and dispelling common misconceptions.
Defining Bases: More Than Just a Bitter Taste
While a bitter taste might be associated with some bases, it's not a defining property. Instead, we rely on several precise definitions and observations to identify a substance as a base. Let's examine the most common definitions:
1. Arrhenius Definition: The Hydroxide Ion Connection
The Arrhenius definition, while limited, provides a good starting point. According to Arrhenius, a base is a substance that increases the concentration of hydroxide ions (OH⁻) when dissolved in water. This increase in hydroxide ions leads to an increase in the solution's pH, making it more alkaline. Classic examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH), which readily dissociate in water to release OH⁻ ions.
Equation: NaOH(aq) → Na⁺(aq) + OH⁻(aq)
2. Brønsted-Lowry Definition: Proton Acceptors
The Brønsted-Lowry definition offers a broader perspective. It defines a base as a proton (H⁺) acceptor. This means a base can receive a proton from an acid. This definition doesn't restrict bases to aqueous solutions, expanding its applicability. For example, ammonia (NH₃) acts as a Brønsted-Lowry base by accepting a proton from water:
Equation: NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Notice how this reaction also produces hydroxide ions, connecting the Brønsted-Lowry and Arrhenius definitions in aqueous solutions. However, the Brønsted-Lowry definition encompasses more substances that act as bases even without directly producing hydroxide ions.
3. Lewis Definition: Electron Pair Donors
The Lewis definition provides the most comprehensive understanding of basicity. A Lewis base is defined as an electron pair donor. This definition encompasses all Brønsted-Lowry bases and extends to many other substances that may not fit the previous definitions. A Lewis base donates a lone pair of electrons to an electron-deficient species (a Lewis acid).
For instance, ammonia (NH₃) acts as a Lewis base because it has a lone pair of electrons on the nitrogen atom that can be donated. The reaction with a Lewis acid like boron trifluoride (BF₃) illustrates this:
Equation: NH₃ + BF₃ → NH₃BF₃
Here, the nitrogen atom in ammonia donates its lone pair to the boron atom in BF₃, forming a coordinate covalent bond. This broader definition significantly expands the scope of base chemistry.
Key Properties of Bases: A Comprehensive Overview
Now that we have established different definitions, let's explore the characteristic properties often associated with bases:
1. pH Greater Than 7: The Alkaline Nature
A crucial property of bases is their pH greater than 7. The pH scale measures the acidity or alkalinity of a solution. A pH of 7 indicates neutrality, while values above 7 indicate increasing alkalinity (basicity). The higher the pH, the stronger the base. This is directly linked to the concentration of hydroxide ions in the solution.
2. Bitter Taste: A Subjective, but Common Association
While not a definitive property for identification, many bases possess a bitter taste. However, it is crucial to avoid tasting any unknown chemical, as many bases can be corrosive and harmful. This observation should only be considered within a controlled laboratory setting with proper safety precautions and only with substances known to be mildly basic.
3. Slippery Feel: A Tactile Indication (with Caution)
Similar to the bitter taste, many bases exhibit a slippery or soapy feel when touched. This is because they react with oils and fats on the skin, producing soap-like substances. Again, it's imperative to avoid touching unknown chemicals due to potential harm.
4. React with Acids to Form Salts and Water: Neutralization Reactions
One of the most significant defining reactions of bases is their reaction with acids. This is known as a neutralization reaction. In this reaction, an acid and a base react to produce a salt and water. This is a hallmark of acid-base chemistry.
General Equation: Acid + Base → Salt + Water
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O):
Equation: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
The neutralization reaction is exothermic, releasing heat, making the solution warmer. The specific salt produced depends on the acid and base used.
5. Change the Color of Indicators: A Visual Cue
Bases can change the color of acid-base indicators. These indicators are substances that change color depending on the pH of the solution. For example, litmus paper turns blue in the presence of a base, while phenolphthalein turns pink. This color change provides a visual method for identifying bases.
6. Conduct Electricity: Due to Ion Formation
Many bases are electrolytes, meaning they can conduct electricity when dissolved in water. This is because bases dissociate into ions in solution, allowing the flow of electric current. The stronger the base, the greater its ability to conduct electricity due to the higher concentration of ions.
7. Catalytic Activity: Specific Bases in Reactions
Certain bases act as catalysts in various chemical reactions. A catalyst speeds up a reaction without being consumed in the process. For instance, many base-catalyzed reactions are used in organic chemistry, such as esterification and saponification (soap-making).
Distinguishing Bases from Acids: A Comparative Analysis
Understanding the properties of bases necessitates a comparison with acids. Here's a table highlighting the key differences:
| Property | Bases | Acids |
|---|---|---|
| Taste | Bitter (with caution) | Sour |
| Feel | Slippery/Soapy (with caution) | Often not distinctive, can be corrosive |
| pH | Greater than 7 | Less than 7 |
| Reaction with Acids | Neutralization; Salt + Water | Neutralization; Salt + Water |
| Effect on Indicators | Changes indicator color (e.g., litmus turns blue) | Changes indicator color (e.g., litmus turns red) |
| Conductivity | Conducts electricity (if dissolved) | Conducts electricity (if dissolved) |
Applications of Bases: A Wide Range of Uses
Bases are essential components in numerous applications across various industries:
-
Industrial Processes: Bases are crucial in manufacturing processes, including the production of soaps, detergents, fertilizers, and paper. They are also used in the refining of metals and in wastewater treatment.
-
Agriculture: Bases are used to adjust the pH of soil, making it suitable for plant growth. They are also components of many fertilizers.
-
Medicine: Many medications contain bases or utilize base-catalyzed reactions in their synthesis. Antacids, for example, use bases to neutralize stomach acid.
-
Household Cleaning: Many household cleaning products contain bases, which help to remove grease and dirt.
-
Food Industry: Some bases are used as food additives or in food processing. They may act as stabilizers, preservatives, or pH regulators.
Conclusion: A Deeper Understanding of Base Chemistry
The properties of bases are multifaceted, extending beyond a simple definition. Understanding the Arrhenius, Brønsted-Lowry, and Lewis definitions provides a comprehensive perspective. While a bitter taste and slippery feel are common associations, they are not reliable identification methods. The key properties include a pH greater than 7, reaction with acids to form salts and water, color changes of indicators, and conductivity of electricity when dissolved. Bases are integral components in numerous industrial, agricultural, and household applications. Careful handling is essential due to the corrosive nature of many strong bases. This in-depth exploration highlights the crucial role of bases in chemistry and their wide-ranging importance in our daily lives.
Latest Posts
Latest Posts
-
How Many Light Years To The Moon
Mar 25, 2025
-
What Is The Correct Order Of Phases In Cellular Respiration
Mar 25, 2025
-
Vessels That Contain Valves To Prevent Backflow Of Blood
Mar 25, 2025
-
Sr Oh 2 Strong Or Weak
Mar 25, 2025
-
What Part Of The Cell Serves As The Intracellular Highway
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Property Of Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
