Sr Oh 2 Strong Or Weak
News Leon
Mar 25, 2025 · 5 min read
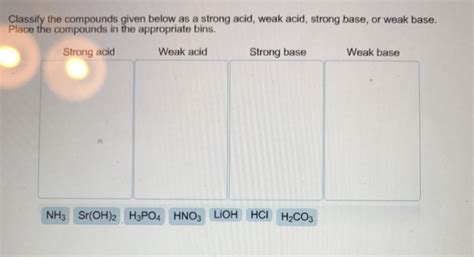
Table of Contents
Sr(OH)₂: Strong or Weak? Understanding its Properties and Applications
Determining whether strontium hydroxide, Sr(OH)₂, is a strong or weak base is crucial for understanding its behavior in chemical reactions and its various applications. This comprehensive guide delves deep into the properties of Sr(OH)₂, exploring its solubility, dissociation, and its practical uses, ultimately answering the question: is Sr(OH)₂ a strong or weak base?
Defining Strong and Weak Bases
Before we delve into the specifics of strontium hydroxide, let's establish a clear understanding of what constitutes a strong base versus a weak base. A strong base is a substance that completely dissociates into its ions (cations and hydroxide anions, OH⁻) when dissolved in water. This means that essentially all of the base molecules break apart, releasing a high concentration of hydroxide ions. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
A weak base, on the other hand, only partially dissociates in water. A significant portion of the base molecules remain intact, resulting in a lower concentration of hydroxide ions compared to a strong base of the same concentration. Ammonia (NH₃) is a classic example of a weak base. The extent of dissociation is quantified by the base dissociation constant, Kb. A higher Kb value indicates a stronger base.
The Solubility of Strontium Hydroxide: A Key Factor
The solubility of Sr(OH)₂ plays a crucial role in determining its strength as a base. While it's often categorized as a strong base, its solubility in water is relatively low compared to strong bases like NaOH or KOH. This limited solubility directly impacts the concentration of hydroxide ions available in solution. It's important to distinguish between strength and concentration. Sr(OH)₂ is strong because when it does dissolve, it fully dissociates. However, because it's not very soluble, its actual concentration of hydroxide ions might be lower than a less soluble but equally strong base.
Understanding Solubility Products (Ksp)
The solubility of Sr(OH)₂ is characterized by its solubility product constant, Ksp. Ksp represents the equilibrium constant for the dissolution of a sparingly soluble salt. A lower Ksp value indicates lower solubility. The Ksp for Sr(OH)₂ is relatively small, reflecting its limited solubility in water. This doesn't mean it's a weak base – it simply means that less of it dissolves, and thus fewer hydroxide ions are produced compared to a highly soluble strong base.
Dissociation of Strontium Hydroxide
The dissociation of strontium hydroxide in water can be represented by the following equation:
Sr(OH)₂(s) ⇌ Sr²⁺(aq) + 2OH⁻(aq)
This equation shows that when Sr(OH)₂ dissolves, it completely dissociates into strontium ions (Sr²⁺) and hydroxide ions (OH⁻). This complete dissociation is the defining characteristic of a strong base. However, the amount that dissociates is limited by its low solubility.
Practical Applications of Strontium Hydroxide
Despite its limited solubility, strontium hydroxide finds various applications in different fields:
1. Sugar Refining:
Sr(OH)₂ has been used in the beet sugar refining process. Its ability to form soluble saccharates with sucrose allows for the separation of sucrose from impurities, leading to a purer sugar product. This application exploits the unique interaction between Sr(OH)₂ and sugar molecules.
2. Lubricant Additives:
Sr(OH)₂ can be used as an additive in lubricants, enhancing their performance and stability. This application often involves specialized formulations and takes advantage of its chemical properties to improve the overall performance of the lubricant system.
3. Chemical Synthesis:
Sr(OH)₂ acts as a source of hydroxide ions in various chemical synthesis reactions. Its use in specific reactions is based on its strong basicity and the unique chemical properties it can impart.
4. Production of other Strontium Compounds:
Sr(OH)₂ serves as a precursor for the synthesis of various other strontium compounds. This underscores its importance in the production chain of different strontium-based materials.
5. Environmental Applications:
While less common, there are niche applications that take advantage of strontium hydroxide's unique chemical behavior in specific environmental contexts. This might involve selective reactions or specific pH adjustments.
Comparing Sr(OH)₂ to Other Bases
To further illustrate the position of Sr(OH)₂ in the spectrum of bases, let's compare it to some other well-known examples:
-
NaOH (Sodium Hydroxide): NaOH is a highly soluble strong base. It completely dissociates in water, producing a high concentration of hydroxide ions. Its high solubility leads to a significantly higher concentration of OH⁻ ions compared to Sr(OH)₂ even though both are strong bases.
-
KOH (Potassium Hydroxide): Similar to NaOH, KOH is a highly soluble strong base. It exhibits complete dissociation and a high concentration of OH⁻ ions in solution.
-
Mg(OH)₂ (Magnesium Hydroxide): Mg(OH)₂ is a weak base with low solubility. While it is considered a weak base because the percentage that dissociates is less than Sr(OH)2, it demonstrates that low solubility doesn't always correlate directly with weak base strength.
The Nuances of Classifying Sr(OH)₂
While the complete dissociation of Sr(OH)₂ when dissolved makes it a strong base, its limited solubility complicates a simple strong/weak categorization. It’s more accurate to say that Sr(OH)₂ is a strong base with low solubility. This distinction is important because it highlights that while it fully dissociates (the hallmark of a strong base), the amount that actually dissociates and contributes to the hydroxide ion concentration is limited by its solubility.
Conclusion: Addressing the Question Directly
So, is Sr(OH)₂ a strong or weak base? The answer is nuanced: Sr(OH)₂ is a strong base, meaning it completely dissociates when dissolved. However, its low solubility limits the concentration of hydroxide ions available in solution. This distinction is crucial for accurate chemical calculations and understanding its behavior in various applications. The limited solubility should not be confused with weak base behavior; it's a separate property that affects the practical concentration of hydroxide ions in a given solution. Understanding both the strong base nature and low solubility is critical for accurately predicting its reactivity and designing applications. The context of the application will dictate how impactful the solubility becomes in determining its usefulness.
Latest Posts
Latest Posts
-
Energy Flow In An Ecosystem Begins With
Mar 25, 2025
-
How Many Moles Of Water Are In 1 Liter
Mar 25, 2025
-
Which One Of The Following Is Not A Strong Electrolyte
Mar 25, 2025
-
In The Figure A Small Nonconducting Ball Of Mass
Mar 25, 2025
-
What Does Rstrip Do In Python
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Sr Oh 2 Strong Or Weak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
