What Are Two Subatomic Particles Found In The Nucleus
News Leon
Mar 22, 2025 · 6 min read
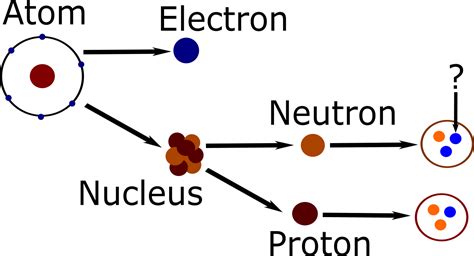
Table of Contents
What Are the Two Subatomic Particles Found in the Nucleus?
The atom, once considered the fundamental building block of matter, is now understood to be a complex system composed of even smaller particles. At the heart of this system lies the nucleus, a dense region containing the majority of the atom's mass. Within this tiny space reside two primary types of subatomic particles: protons and neutrons. Understanding their properties, interactions, and significance is crucial to grasping the fundamentals of chemistry, physics, and nuclear science.
Protons: The Positively Charged Guardians
Protons are subatomic particles carrying a single positive electric charge (+1e, where 'e' represents the elementary charge). This positive charge is fundamental to their role in the atom. Crucially, the number of protons in an atom's nucleus defines its atomic number, which uniquely identifies the element. For example, an atom with one proton is hydrogen (atomic number 1), while an atom with six protons is carbon (atomic number 6). This number is unwavering; it's what fundamentally distinguishes one element from another.
Properties of Protons:
- Charge: +1e
- Mass: Approximately 1.6726 × 10^-27 kg (This is roughly 1836 times the mass of an electron).
- Spin: 1/2 (This is a quantum property related to intrinsic angular momentum).
- Composition: Protons are themselves composed of even smaller particles called quarks – specifically, two up quarks and one down quark. The interaction of these quarks, mediated by gluons (the force-carrying particles of the strong nuclear force), holds the proton together.
- Stability: Protons are remarkably stable particles. While they can participate in nuclear reactions, they don't spontaneously decay under normal conditions. This stability is paramount to the stability of atoms themselves.
The Role of Protons in Chemical Reactions:
The positive charge of protons plays a pivotal role in chemical bonding. The outermost electrons of an atom, which are relatively loosely bound to the nucleus, are attracted to the positive charge of the protons in other atoms. This electrostatic attraction is the foundation of chemical bonds, such as ionic and covalent bonds, which hold atoms together to form molecules and compounds. Understanding the number of protons and the arrangement of electrons is therefore key to understanding chemical reactivity and the behavior of matter.
Neutrons: The Neutral Stabilizers
Neutrons, as their name suggests, are electrically neutral particles. They carry no electric charge (0e). While they don't directly participate in chemical bonding (because they have no charge to interact electrostatically), their presence in the nucleus is crucial for nuclear stability and the existence of most isotopes.
Properties of Neutrons:
- Charge: 0e
- Mass: Approximately 1.6749 × 10^-27 kg (slightly larger than the mass of a proton).
- Spin: 1/2
- Composition: Like protons, neutrons are also composed of quarks – one up quark and two down quarks.
- Stability: Free neutrons are unstable and undergo beta decay, transforming into a proton, an electron, and an antineutrino, with a half-life of about 10 minutes. However, neutrons within the nucleus are generally stable, except in certain radioactive isotopes where the neutron-proton ratio is imbalanced.
The Role of Neutrons in Nuclear Stability:
The number of neutrons in a nucleus significantly impacts its stability. For lighter elements, the number of neutrons is roughly equal to the number of protons. However, as the atomic number increases, the number of neutrons required for stability exceeds the number of protons. This is because the strong nuclear force, which holds the nucleus together, is short-ranged. Neutrons help to overcome the electrostatic repulsion between positively charged protons, thereby contributing to the overall stability of the nucleus. Isotopes of the same element have the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive, meaning their nuclei are unstable and decay over time, emitting radiation in the process.
The Strong Nuclear Force: The Glue Holding the Nucleus Together
The incredible density of the nucleus, where positively charged protons are crammed together, might seem counterintuitive. After all, like charges repel. The strong nuclear force is the fundamental force responsible for overcoming this electrostatic repulsion and holding the nucleus together. This force is significantly stronger than the electromagnetic force at very short distances, but its influence diminishes rapidly with distance. The strong nuclear force acts between protons and neutrons, mediated by gluons. The balance between the strong nuclear force and the electromagnetic force determines the stability or instability of a nucleus.
Isotopes and Nuclear Stability: The Neutron-Proton Ratio
The ratio of neutrons to protons in a nucleus significantly influences its stability. A stable nucleus typically has a neutron-to-proton ratio close to 1 for lighter elements. However, this ratio increases for heavier elements, exceeding 1.5 in some cases. This is because the increasing number of protons necessitates a greater number of neutrons to counteract the growing electrostatic repulsion. When the neutron-to-proton ratio deviates significantly from the optimal value, the nucleus becomes unstable and undergoes radioactive decay.
Radioactive Decay: When the Nucleus Becomes Unstable
Radioactive decay is a process by which unstable atomic nuclei lose energy by emitting radiation. This radiation can take various forms, including alpha particles (helium nuclei), beta particles (electrons or positrons), and gamma rays (high-energy photons). The type of radiation emitted and the rate of decay are characteristic of the specific radioactive isotope. Radioactive decay has numerous applications in various fields, including medicine, archaeology, and industrial processes.
Types of Radioactive Decay:
- Alpha Decay: The emission of an alpha particle (two protons and two neutrons), reducing the atomic number by 2 and the mass number by 4.
- Beta Decay: The emission of a beta particle (an electron or a positron), changing a neutron into a proton (or vice-versa).
- Gamma Decay: The emission of a gamma ray (high-energy photon), which doesn't change the atomic number or mass number but reduces the energy of the nucleus.
Nuclear Fusion and Fission: Harnessing Nuclear Power
The properties of protons and neutrons are central to understanding nuclear reactions, which involve changes in the composition of atomic nuclei. Two prominent types of nuclear reactions are nuclear fusion and nuclear fission.
Nuclear Fusion: Combining Nuclei
Nuclear fusion is the process of combining two or more atomic nuclei to form a heavier nucleus. This process releases a tremendous amount of energy because the mass of the resulting nucleus is slightly less than the sum of the masses of the original nuclei. This mass difference is converted into energy according to Einstein's famous equation, E=mc². Fusion reactions power the sun and other stars, releasing immense energy.
Nuclear Fission: Splitting Nuclei
Nuclear fission is the process of splitting a heavy atomic nucleus into two or more smaller nuclei. This process also releases a significant amount of energy, as the mass of the resulting nuclei is less than the mass of the original nucleus. Nuclear fission is the basis of nuclear power plants and nuclear weapons. The controlled fission of uranium-235 or plutonium-239 is used to generate electricity in nuclear power plants.
Conclusion: The Foundation of Matter
Protons and neutrons, the two subatomic particles residing within the nucleus, are fundamental components of matter. Their properties – charge, mass, and interactions via the strong nuclear force – dictate the behavior of atoms, the formation of molecules, and the possibility of nuclear reactions. Understanding these particles and their roles is crucial for advancing our knowledge of chemistry, physics, and the universe around us. From the smallest atoms to the largest stars, the behavior of protons and neutrons governs the structure and processes of the cosmos. Further research into their properties and interactions continues to push the boundaries of scientific understanding, unveiling new insights into the fundamental building blocks of our reality.
Latest Posts
Latest Posts
-
1 1 Sin 1 1 Sin
Mar 22, 2025
-
Oxidation Number For Cr In Cr2o72
Mar 22, 2025
-
Which Of The Following Statements About Surfactants Is Not True
Mar 22, 2025
-
How Long Was Rip Van Winkle Asleep
Mar 22, 2025
-
What Is 5 Percent Of 600
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Are Two Subatomic Particles Found In The Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
