The Two Strands Of Dna Are Held Together By
News Leon
Mar 28, 2025 · 6 min read
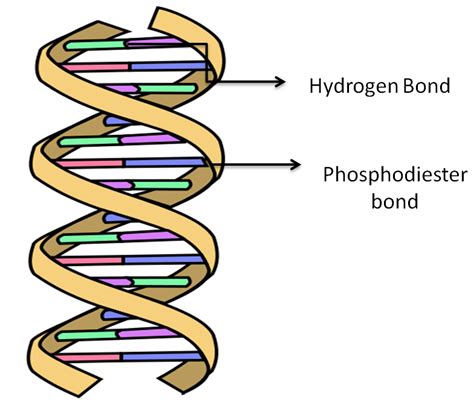
Table of Contents
The Two Strands of DNA: A Deep Dive into the Bonds that Hold Life Together
Deoxyribonucleic acid, or DNA, is the fundamental blueprint of life. This remarkable molecule, found in almost all living organisms, holds the genetic instructions for development, functioning, growth, and reproduction. But the beauty of DNA isn't just in the information it encodes; it's also in the elegant and precise way it's structured. This article delves deep into the fascinating world of DNA structure, focusing specifically on the crucial bonds that hold its two strands together: hydrogen bonds. We'll explore their properties, significance, and the implications of their strength and specificity for life itself.
The Double Helix: A Masterpiece of Molecular Architecture
The iconic double helix structure of DNA, discovered by Watson and Crick, is a testament to the power of elegant simplicity. Two polynucleotide strands, each composed of a sugar-phosphate backbone and a series of nitrogenous bases, intertwine to form a twisting ladder-like structure. This structure isn't simply a random arrangement; it's stabilized by a network of precisely positioned hydrogen bonds that connect the bases on opposite strands.
Understanding the Building Blocks: Nucleotides and Bases
Before we explore the bonds, let's revisit the fundamental components of DNA: nucleotides. Each nucleotide consists of three parts:
- A deoxyribose sugar: A five-carbon sugar molecule forming the backbone of the strand.
- A phosphate group: Connects the sugar molecules in the backbone, creating a negatively charged polymer.
- A nitrogenous base: One of four molecules – adenine (A), guanine (G), cytosine (C), and thymine (T) – that carry the genetic information.
These bases are the key players in the hydrogen bonding that holds the DNA strands together. Their specific chemical structures dictate which bases can pair with each other and how many hydrogen bonds are formed.
Hydrogen Bonds: The Glue that Holds DNA Together
The two strands of DNA aren't held together by strong covalent bonds, which would be too rigid and prevent the DNA from performing its essential functions, such as replication and transcription. Instead, they are held together by weaker yet vital hydrogen bonds that form between the nitrogenous bases.
Hydrogen bonds are a special type of dipole-dipole attraction between a hydrogen atom bonded to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom. This results in a partially positive hydrogen atom being attracted to a partially negative atom on another molecule. In DNA, these interactions occur between the bases:
- Adenine (A) always pairs with Thymine (T): They are connected by two hydrogen bonds.
- Guanine (G) always pairs with Cytosine (C): They are connected by three hydrogen bonds.
This specific base pairing, known as Chargaff's rules, is fundamental to DNA's structure and function. The complementary nature of base pairing ensures that the genetic information is accurately replicated and transcribed.
The Strength and Specificity of Hydrogen Bonds in DNA
While individually weak, the sheer number of hydrogen bonds in a DNA molecule collectively provides significant stability. The cumulative effect of many hydrogen bonds along the DNA strands contributes to the overall strength of the double helix. However, the relative weakness of individual hydrogen bonds is crucial because it allows the DNA strands to separate during replication and transcription. Enzymes can easily break these bonds, allowing access to the genetic information encoded within.
The specificity of hydrogen bonding is equally important. The precise shapes and chemical properties of the bases ensure that only A pairs with T and G pairs with C. This precise pairing is vital for maintaining the integrity of the genetic code and preventing errors during replication. Any mismatch would lead to mutations that could have significant consequences for the organism.
Beyond Hydrogen Bonds: Other Forces Stabilizing the DNA Double Helix
While hydrogen bonds are the primary force holding the DNA strands together, other interactions also contribute to the stability of the double helix:
- Hydrophobic interactions: The bases are relatively hydrophobic (water-repelling), and they stack together within the interior of the helix, minimizing their contact with water. This stacking contributes significantly to the stability of the structure.
- Van der Waals forces: These weak, short-range forces arise from temporary fluctuations in electron distribution around the molecules. Although individually weak, the cumulative effect of these forces along the DNA strand contributes to overall stability.
- Ionic interactions: The negatively charged phosphate groups in the DNA backbone repel each other. However, this repulsion is counteracted by the positive charges of ions in the surrounding environment, such as magnesium ions (Mg²⁺), which help to stabilize the DNA structure.
These interactions, in conjunction with the hydrogen bonds, create a remarkably stable yet dynamic structure that allows DNA to perform its vital functions.
The Significance of Hydrogen Bonds in DNA Replication and Transcription
The ability of hydrogen bonds to be relatively easily broken and reformed is absolutely critical for DNA's two major functions: replication and transcription.
DNA Replication: Duplicating the Genetic Code
During DNA replication, the two strands of the DNA double helix separate, allowing each strand to serve as a template for the synthesis of a new complementary strand. The hydrogen bonds between the base pairs are broken by enzymes called helicases. Then, DNA polymerase, another crucial enzyme, adds new nucleotides to each template strand, following the rules of base pairing (A with T and G with C). The newly formed hydrogen bonds between the base pairs of the newly synthesized strands and the template strands stabilize the two newly formed DNA double helices.
DNA Transcription: From DNA to RNA
Transcription is the process by which the genetic information encoded in DNA is copied into a messenger RNA (mRNA) molecule. Similar to replication, the hydrogen bonds between the DNA base pairs are broken, allowing RNA polymerase to access the DNA template strand. The RNA polymerase then synthesizes a complementary mRNA molecule, using the DNA template strand as a guide. The mRNA molecule carries the genetic information from the DNA to the ribosomes, where it directs protein synthesis.
Consequences of Disruptions to Hydrogen Bonding in DNA
The stability of the DNA double helix relies heavily on the integrity of the hydrogen bonds. Any disruption to these bonds can lead to serious consequences:
- Mutations: Errors during DNA replication, such as mismatched base pairs, can result from weakened or disrupted hydrogen bonds. These errors can lead to mutations, which can have various effects on the organism, ranging from benign to harmful.
- DNA Damage: Exposure to certain chemicals or radiation can damage DNA, leading to the breaking of hydrogen bonds and potentially leading to strand breakage or other structural abnormalities. These types of damage can have serious health consequences.
- Diseases: Several diseases are linked to problems with DNA structure or replication, often due to issues related to hydrogen bonding or interactions affecting DNA stability.
Conclusion: A Delicate Balance of Strength and Weakness
The hydrogen bonds holding the two strands of DNA together are a marvel of biological engineering. Their relatively weak nature allows for the essential processes of DNA replication and transcription, while their collective strength ensures the stability of the genetic code. Understanding the intricate balance of forces that maintain DNA's structure is crucial for comprehending the fundamental mechanisms of life itself. Further research into the dynamics of hydrogen bonding and other stabilizing forces within DNA will continue to deepen our understanding of this fundamental molecule and its role in health and disease. The precise nature of these bonds, their susceptibility to disruption, and the cellular mechanisms that maintain and repair them remain active areas of research with significant implications for medicine and biotechnology.
Latest Posts
Latest Posts
-
Which Type Of Muscle Tissue Is Multinucleated
Mar 31, 2025
-
True Or False Evaporation Is A Physical Change
Mar 31, 2025
-
Do Gram Positive Bacteria Have Porins
Mar 31, 2025
-
Which Of The Following Compounds Is Most Soluble In Water
Mar 31, 2025
-
Part Of The Brain That Controls Breathing And Heartbeat
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Two Strands Of Dna Are Held Together By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
