Packing Efficiency Of Body Centered Cubic
News Leon
Mar 19, 2025 · 6 min read
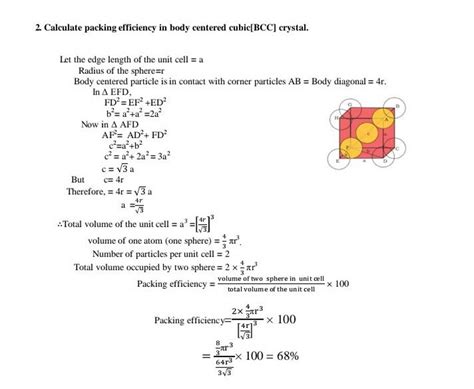
Table of Contents
Packing Efficiency of Body-Centered Cubic (BCC) Structures
The arrangement of atoms within a crystal structure significantly influences its physical properties, including density, strength, and conductivity. One common crystal structure is the body-centered cubic (BCC) structure, characterized by its unique arrangement of atoms and resulting packing efficiency. Understanding the packing efficiency of BCC structures is crucial in materials science, enabling us to predict and manipulate the properties of various materials. This article delves into the intricacies of BCC packing, calculating its efficiency, and comparing it to other common crystal structures.
Understanding the Body-Centered Cubic (BCC) Structure
In a BCC structure, each atom sits at the corners of a cube, and a single atom occupies the center of the cube. This central atom is surrounded by eight corner atoms. This arrangement contrasts with other structures like simple cubic (SC) and face-centered cubic (FCC), which have different atomic distributions.
Visualizing the BCC Structure
Imagine a cube. At each of the eight corners of this cube, you place an atom. Now, place a single atom precisely in the center of this cube. This arrangement perfectly depicts a BCC unit cell. It's important to understand that this unit cell is a repeating unit that builds the entire crystal structure. The atoms touch along the body diagonal, but not along the edges or faces of the cube.
Atomic Coordination Number in BCC
The atomic coordination number refers to the number of nearest neighbors surrounding a given atom. In a BCC structure, each atom is surrounded by eight nearest neighbors—the eight corner atoms of the cube. This leads to a coordination number of 8.
Calculating the Packing Efficiency of BCC
The packing efficiency describes the proportion of space within a unit cell occupied by atoms, expressed as a percentage. It's a crucial parameter for understanding the density of a material. Let's calculate the packing efficiency of the BCC structure step-by-step:
1. Number of Atoms per Unit Cell
The BCC unit cell contains a total of two atoms:
- Corner atoms: Each of the eight corner atoms is shared by eight adjacent unit cells. Therefore, each unit cell “owns” only 1/8 of each corner atom. This contributes 8 * (1/8) = 1 atom.
- Center atom: The atom at the center of the cube belongs entirely to the unit cell. This adds another 1 atom.
Thus, a BCC unit cell contains 2 atoms.
2. Volume of Atoms in the Unit Cell
Let's assume the radius of each atom is 'r'. The atoms in a BCC structure touch along the body diagonal of the cube. The length of the body diagonal (d) can be related to the side length (a) of the cube using the Pythagorean theorem in three dimensions:
d² = a² + a² + a² = 3a² => d = a√3
Since the atoms touch along the body diagonal, the length of the body diagonal is equal to four times the atomic radius (4r):
4r = a√3
Solving for 'a', we get:
a = 4r / √3
The volume of the unit cell (V<sub>cell</sub>) is simply a³:
V<sub>cell</sub> = a³ = (4r / √3)³ = 64r³ / 3√3
The volume of a single atom (V<sub>atom</sub>) is (4/3)πr³
The total volume of atoms in the unit cell (V<sub>atoms</sub>) is the volume of two atoms:
V<sub>atoms</sub> = 2 * (4/3)πr³ = (8/3)πr³
3. Calculating Packing Efficiency
The packing efficiency (η) is the ratio of the volume occupied by atoms to the total volume of the unit cell, expressed as a percentage:
η = (V<sub>atoms</sub> / V<sub>cell</sub>) * 100%
Substituting the values we derived:
η = [(8/3)πr³ / (64r³ / 3√3)] * 100%
Simplifying, we get:
η = (√3 * π) / 8 * 100% ≈ 68%
Therefore, the packing efficiency of a BCC structure is approximately 68%. This means that approximately 68% of the space within a BCC unit cell is occupied by atoms, while the remaining 32% is empty space.
Comparison with Other Crystal Structures
Let's compare the packing efficiency of BCC with other common crystal structures:
-
Simple Cubic (SC): The SC structure has a packing efficiency of only 52%. This is because the atoms are only in contact along the edges of the cube and do not touch along the diagonals.
-
Face-Centered Cubic (FCC): The FCC structure has a significantly higher packing efficiency of 74%. This is due to the more efficient arrangement of atoms in the FCC unit cell. The atoms touch along the face diagonals.
Significance of Packing Efficiency in Material Properties
The packing efficiency directly influences several material properties:
-
Density: Materials with higher packing efficiency tend to have higher densities. This is because more atoms are packed into the same volume.
-
Mechanical Strength: A higher packing efficiency generally results in increased strength. This is because the atoms are more closely packed together, leading to stronger interatomic forces.
-
Electrical Conductivity: Packing efficiency can influence electrical conductivity in metals. Closely packed structures generally exhibit better electrical conductivity.
-
Thermal Conductivity: Similar to electrical conductivity, thermal conductivity is influenced by atomic packing. Higher packing efficiency tends to lead to better thermal conductivity.
Applications of BCC Structures
Many metals and alloys exhibit a BCC structure at certain temperatures. Some examples include:
-
Iron (α-iron): At room temperature, iron adopts a BCC structure.
-
Chromium: Chromium is another example of a metal that has a BCC structure.
-
Tungsten: Tungsten, known for its high melting point, also possesses a BCC structure.
-
Molybdenum: Similar to tungsten, molybdenum also exhibits a BCC structure.
The properties of these materials are directly related to their BCC structure and its associated packing efficiency.
Advanced Concepts and Further Exploration
The discussion above provides a foundational understanding of BCC packing efficiency. However, there are more advanced concepts to consider:
-
Interstitial Sites: BCC structures contain interstitial sites, which are spaces between the atoms. These sites can accommodate smaller atoms, leading to interstitial alloys.
-
Temperature Dependence: The crystal structure of some materials can change with temperature. For example, iron transitions from BCC to FCC at higher temperatures.
-
Deformation Mechanisms: Understanding the packing efficiency is vital to comprehending how BCC materials deform under stress. The relatively lower packing efficiency compared to FCC structures can influence the deformation mechanisms.
-
Computational Modeling: Advanced computational techniques such as molecular dynamics and density functional theory are used to study the intricate atomic arrangements and properties of BCC structures in detail.
Conclusion
The body-centered cubic structure, with its unique atomic arrangement, presents a packing efficiency of approximately 68%. While this is lower than the FCC structure's 74%, it is significantly higher than the simple cubic structure's 52%. This packing efficiency, along with the coordination number and interstitial sites, directly influences a wide range of material properties such as density, strength, conductivity, and deformation behavior. Understanding the BCC structure is critical for materials scientists and engineers in designing and developing materials with specific properties for various applications. Further research and exploration into the intricacies of BCC structures, particularly using computational techniques, promise deeper insights into its fascinating properties and potential applications.
Latest Posts
Latest Posts
-
The Solubility Of A Solute Depends On
Mar 19, 2025
-
The Complete Set Of Genes In An Organism
Mar 19, 2025
-
Formula Of Coefficient Of Kinetic Friction
Mar 19, 2025
-
What Are The Three Parts Of The Atp Molecule
Mar 19, 2025
-
Whether A Molecule Can Cross The Plasma Membrane Depends Upon
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Packing Efficiency Of Body Centered Cubic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
