Oxidation Number Of Cr In Cr2o72
News Leon
Mar 19, 2025 · 6 min read
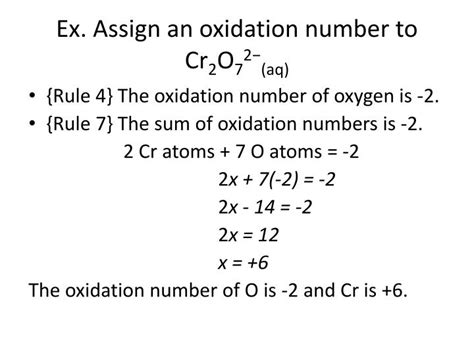
Table of Contents
Determining the Oxidation Number of Cr in Cr₂O₇²⁻: A Comprehensive Guide
The dichromate ion, Cr₂O₇²⁻, is a powerful oxidizing agent frequently encountered in chemistry, particularly in redox reactions. Understanding its structure and, more importantly, the oxidation number of chromium (Cr) within the ion is crucial for predicting its reactivity and balancing redox equations. This article delves deep into the process of determining the oxidation number of Cr in Cr₂O₇²⁻, covering various aspects and providing a comprehensive understanding of the concept.
Understanding Oxidation Numbers
Before we tackle the specific case of Cr₂O₇²⁻, let's establish a solid foundation in understanding oxidation numbers. The oxidation number, also known as the oxidation state, is a number assigned to an atom in a chemical compound that represents the hypothetical charge the atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial concept in redox chemistry, helping us track electron transfer during chemical reactions.
Several rules govern the assignment of oxidation numbers:
- Rule 1: The oxidation number of an element in its free or uncombined state is always zero. For example, the oxidation number of O₂ is 0, and the oxidation number of Fe in a piece of iron is 0.
- Rule 2: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
- Rule 3: The sum of the oxidation numbers of all atoms in a neutral molecule is zero.
- Rule 4: The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. This is the key rule for determining the oxidation number of Cr in Cr₂O₇²⁻.
- Rule 5: The oxidation number of hydrogen is +1, except in metal hydrides where it is -1.
- Rule 6: The oxidation number of oxygen is usually -2, except in peroxides (like H₂O₂) where it is -1 and in compounds with fluorine where it can be positive.
- Rule 7: The oxidation number of alkali metals (Group 1) is +1.
- Rule 8: The oxidation number of alkaline earth metals (Group 2) is +2.
- Rule 9: The oxidation number of halogens (Group 17) is usually -1, except when combined with oxygen or another halogen with higher electronegativity.
Determining the Oxidation Number of Cr in Cr₂O₇²⁻
Now, let's apply these rules to determine the oxidation number of chromium in the dichromate ion, Cr₂O₇²⁻. We'll use Rule 4, which states that the sum of the oxidation numbers of all atoms in a polyatomic ion equals the charge of the ion.
-
Identify the known oxidation numbers: We know the oxidation number of oxygen is typically -2 (Rule 6).
-
Set up an algebraic equation: Let 'x' represent the oxidation number of chromium (Cr). The dichromate ion has two chromium atoms and seven oxygen atoms. Therefore, the equation becomes:
2x + 7(-2) = -2
-
Solve for x:
2x - 14 = -2 2x = 12 x = +6
Therefore, the oxidation number of chromium (Cr) in Cr₂O₇²⁻ is +6.
The Significance of the +6 Oxidation State of Chromium
The +6 oxidation state of chromium in the dichromate ion is significant for several reasons:
-
Strong Oxidizing Agent: Chromium in the +6 oxidation state is a strong oxidizing agent. This means it readily accepts electrons from other substances, causing them to be oxidized. This property makes Cr₂O₇²⁻ crucial in various redox reactions, including those used in titrations and organic chemistry.
-
Color: The intense orange color of the dichromate ion is a characteristic of chromium in its +6 oxidation state. The color arises from the electronic transitions within the chromium ion.
-
Toxicity: Chromium(VI) compounds, including Cr₂O₇²⁻, are known to be toxic and carcinogenic. Appropriate safety precautions must always be taken when handling these compounds.
-
Applications: The strong oxidizing power of Cr₂O₇²⁻ finds applications in various fields, such as:
- Cleaning solutions: Dichromate is used in cleaning solutions for glassware and other laboratory equipment.
- Leather tanning: The oxidizing properties of chromium are useful in the leather tanning industry.
- Electroplating: Chromium plating often involves the use of chromium(VI) compounds.
- Chemical synthesis: Dichromate is used as an oxidant in various chemical reactions.
Further Exploration of Oxidation Numbers and Redox Reactions
Understanding oxidation numbers is fundamental to understanding redox reactions. Redox reactions, or reduction-oxidation reactions, involve the transfer of electrons between species. Oxidation is the loss of electrons, while reduction is the gain of electrons. In Cr₂O₇²⁻, the high oxidation state of chromium (+6) indicates that it's highly likely to undergo reduction (gain electrons) during a redox reaction.
Balancing redox equations often requires careful consideration of oxidation numbers. The change in oxidation numbers helps determine the stoichiometry of the reaction and the number of electrons transferred. Various methods exist for balancing redox equations, including the half-reaction method and the oxidation number method.
Beyond Cr₂O₇²⁻: Other Chromium Compounds and Oxidation States
Chromium exhibits a variety of oxidation states, including +2, +3, and +6. Each oxidation state has its own characteristic properties and reactivity. For instance:
- Chromium(II) (Cr²⁺): A reducing agent, often present in pale blue solutions.
- Chromium(III) (Cr³⁺): Relatively stable and commonly found in green compounds.
- Chromium(VI) (Cr⁶⁺): A strong oxidizing agent, usually found in yellow or orange compounds, including Cr₂O₇²⁻ and chromate (CrO₄²⁻).
The oxidation state of chromium significantly influences its chemical behavior. Therefore, understanding how to determine oxidation numbers is critical for predicting the reactivity and applications of various chromium compounds.
Practical Applications and Examples
Let's consider a few examples to further illustrate the concept and its practical implications.
Example 1: The reaction of Cr₂O₇²⁻ with Fe²⁺:
In acidic solutions, dichromate ions react with ferrous ions (Fe²⁺) according to the following unbalanced equation:
Cr₂O₇²⁻ + Fe²⁺ → Cr³⁺ + Fe³⁺
To balance this equation, we need to consider the changes in oxidation numbers. Chromium goes from +6 to +3 (reduction), and iron goes from +2 to +3 (oxidation). Balancing this equation requires careful consideration of electron transfer and the use of appropriate stoichiometric coefficients.
Example 2: Determining Oxidation Numbers in Other Compounds:
Consider the compound potassium permanganate (KMnO₄). To determine the oxidation number of manganese (Mn), we use the known oxidation numbers of potassium (+1) and oxygen (-2). This leads to the following equation:
(+1) + x + 4(-2) = 0
Solving for x, we find that the oxidation number of manganese in KMnO₄ is +7.
Conclusion
Determining the oxidation number of Cr in Cr₂O₇²⁻, as we have demonstrated, is a straightforward application of fundamental rules of oxidation number assignment. The resulting +6 oxidation state is crucial for understanding the chemical properties, reactivity, and applications of the dichromate ion. Mastering the concept of oxidation numbers and their application in redox reactions is essential for anyone studying or working in chemistry, providing a key to unlocking the intricacies of chemical transformations and reactions. The information provided in this article serves as a solid foundation for further exploration into the fascinating world of redox chemistry and the diverse roles of chromium compounds in various chemical and industrial processes. Understanding oxidation numbers is not just a theoretical exercise; it is a practical tool for predicting chemical behavior and for designing and optimizing chemical processes.
Latest Posts
Latest Posts
-
The Solubility Of A Solute Depends On
Mar 19, 2025
-
The Complete Set Of Genes In An Organism
Mar 19, 2025
-
Formula Of Coefficient Of Kinetic Friction
Mar 19, 2025
-
What Are The Three Parts Of The Atp Molecule
Mar 19, 2025
-
Whether A Molecule Can Cross The Plasma Membrane Depends Upon
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of Cr In Cr2o72 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
