Orbitals That Have The Same Energy Are Called
News Leon
Mar 31, 2025 · 5 min read
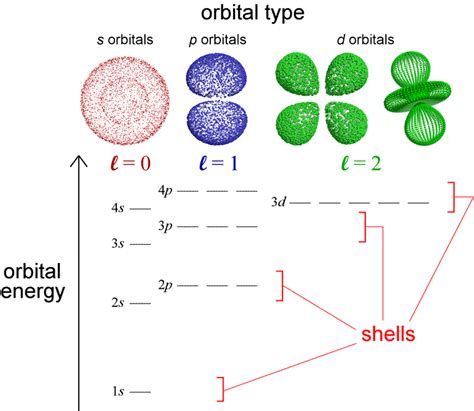
Table of Contents
Orbitals That Have the Same Energy Are Called Degenerate Orbitals: A Deep Dive
Orbitals are regions of space around an atom's nucleus where there's a high probability of finding an electron. Understanding orbital energy levels is crucial to grasping atomic structure, chemical bonding, and the behavior of matter. A fundamental concept in this understanding is degeneracy, specifically, orbitals that have the same energy are called degenerate orbitals. This article will delve deep into the concept of degenerate orbitals, exploring their characteristics, the factors influencing degeneracy, and the consequences of degeneracy removal.
What are Degenerate Orbitals?
Degenerate orbitals are atomic orbitals that possess the same energy level within a given electron shell. This means electrons can occupy any of these orbitals with equal probability. The energy degeneracy arises from the symmetry of the atom's electron cloud. For instance, in a hydrogen atom, the three p-orbitals (px, py, and pz) are degenerate because they have the same energy level. However, this degeneracy is not a universal truth; it's highly dependent on several factors.
Key Characteristics of Degenerate Orbitals:
- Equal Energy: This is the defining characteristic. Electrons in degenerate orbitals have the same energy.
- Different Spatial Orientation: Though they possess the same energy, degenerate orbitals often differ in their spatial orientation. The p-orbitals, for example, point along the x, y, and z axes.
- Same Principal Quantum Number (n): Degenerate orbitals within the same subshell always share the same principal quantum number (n), which determines the energy level of the electron shell.
- Same Azimuthal Quantum Number (l): They also have the same azimuthal quantum number (l), indicating the subshell (s, p, d, f, etc.).
- Different Magnetic Quantum Number (ml): They possess different magnetic quantum numbers (ml), which specifies the orbital's orientation in space.
Factors Affecting Orbital Degeneracy
While the simple picture of degenerate orbitals is helpful, several factors can lift this degeneracy, causing orbitals of the same subshell to have slightly different energies.
1. Electron-Electron Repulsion:
In atoms with multiple electrons, the mutual repulsion between electrons significantly affects orbital energies. This interaction is not considered in the simple hydrogen atom model. Electrons in different orbitals experience varying degrees of shielding from the nuclear charge and repulsion from other electrons. This leads to a slight difference in their effective nuclear charge and consequently, their energies. The higher the number of electrons, the more pronounced the effect of electron-electron repulsion on degeneracy.
2. External Electric or Magnetic Fields:
Applying an external electric field (Stark effect) or a magnetic field (Zeeman effect) can lift the degeneracy of orbitals. These fields interact with the electrons' charge and spin, respectively, causing a splitting of energy levels. The degeneracy is removed, and the orbitals which were once degenerate now have different energy levels.
3. Molecular Geometry and Chemical Bonding:
In molecules, the symmetry of the molecular environment plays a crucial role in determining orbital energies. The interaction between atomic orbitals during bond formation can lead to the splitting of degenerate atomic orbitals into bonding and antibonding molecular orbitals with different energies. This splitting is directly related to the symmetry of the molecule and the types of atomic orbitals involved in the bonding.
4. Nuclear Charge and Shielding Effects:
As the nuclear charge increases across a period in the periodic table, the attraction between the nucleus and electrons strengthens. However, the presence of inner electrons shields the outer electrons from the full nuclear charge. This shielding effect isn't uniform for all orbitals, leading to subtle energy differences between degenerate orbitals, even without external fields or electron-electron repulsion significantly altering things.
5. Relativistic Effects:
For heavier atoms, relativistic effects become significant. The high speed of inner electrons, approaching the speed of light, causes a contraction of their orbitals and a corresponding increase in their energy. This relativistic effect can influence the energy levels of different orbitals differently, removing some degeneracy.
Consequences of Degeneracy Removal:
Lifting degeneracy has significant consequences for chemical behavior and properties.
1. Spectral Line Splitting:
When degeneracy is removed, atomic transitions between different energy levels lead to a splitting of spectral lines. This splitting provides valuable information about the electronic structure and interactions within the atom. The observed splitting patterns can be used to identify the factors responsible for degeneracy removal.
2. Changes in Chemical Reactivity:
The energy differences between formerly degenerate orbitals influence the relative energies of molecular orbitals formed during chemical bonding. This affects bond strength, reactivity, and other chemical properties. Molecules with non-degenerate orbitals can exhibit properties different from those predicted by simple models assuming degeneracy.
3. Differences in Magnetic Properties:
The splitting of orbitals due to magnetic fields (Zeeman effect) is responsible for the magnetic properties of many substances. It underlies various spectroscopic techniques used to study the electronic structure and magnetic properties of matter.
Examples of Degenerate Orbitals
Let's explore some specific examples:
1. Hydrogen Atom:
In a hydrogen atom, the three 2p orbitals (2px, 2py, 2pz) are degenerate in the absence of external fields. They have the same energy but differ in their spatial orientation.
2. Multi-electron Atoms:
In multi-electron atoms, the degeneracy of orbitals is often lifted due to electron-electron interactions. For example, in a carbon atom, the three 2p orbitals are not perfectly degenerate due to electron-electron repulsion.
3. Transition Metal Ions:
Transition metal ions have partially filled d-orbitals. In octahedral complexes, the five d-orbitals are split into two sets of orbitals with different energies due to ligand field effects (removal of degeneracy). This splitting is crucial in determining the color, magnetism, and reactivity of these complexes.
Conclusion:
The concept of degenerate orbitals provides a fundamental framework for understanding atomic and molecular structure. While the idea of orbitals having the same energy is a simplification for many systems, it serves as a critical starting point. Understanding the factors that can lift this degeneracy and the consequences of this removal is crucial for explaining many observed phenomena in chemistry, physics, and materials science. The interplay between electron-electron interactions, external fields, molecular geometry, and relativistic effects significantly impacts the energy levels of orbitals, leading to a richer and more complex understanding of atomic and molecular behavior. Further research continues to refine our understanding of orbital degeneracy and its implications for a wide range of scientific disciplines.
Latest Posts
Latest Posts
-
Count Vowels In A String Python
Apr 01, 2025
-
Which Of The Following Elements Is Most Electronegative
Apr 01, 2025
-
For Which Value Of X Is Abcd A Kite
Apr 01, 2025
-
64 To The Power Of 1 2
Apr 01, 2025
-
On The Galapagos Islands Charles Darwin Observed
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Orbitals That Have The Same Energy Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
