Oil And Water Is What Type Of Mixture
News Leon
Mar 27, 2025 · 6 min read
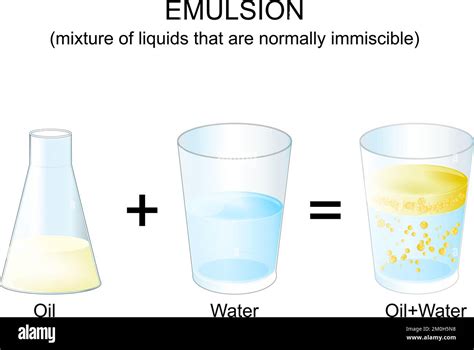
Table of Contents
Oil and Water: An Exploration of Immiscible Mixtures
Oil and water. The classic example of substances that don't mix. But why don't they mix? And what exactly is the type of mixture they form? This seemingly simple question delves into the fascinating world of chemistry, exploring concepts like polarity, intermolecular forces, and the very nature of mixtures themselves. This article will provide a comprehensive overview, exploring the science behind oil and water's incompatibility and classifying their interaction as a specific type of mixture.
Understanding Mixtures: A Fundamental Concept
Before we delve into the specifics of oil and water, let's establish a clear understanding of mixtures. In chemistry, a mixture is a substance comprising two or more components not chemically bonded. Crucially, these components retain their individual chemical properties. This is in contrast to a compound, where components chemically react to form a new substance with different properties.
Mixtures can be further classified into two main categories: homogeneous and heterogeneous.
-
Homogeneous mixtures: These mixtures have a uniform composition throughout. No matter where you take a sample, the proportions of the components will be the same. Examples include saltwater (salt dissolved in water) and air (a mixture of various gases).
-
Heterogeneous mixtures: These mixtures have a non-uniform composition. Different parts of the mixture have different proportions of the components. Examples include sand and water, oil and water, and a salad.
The Polarity Puzzle: Why Oil and Water Don't Mix
The key to understanding why oil and water don't mix lies in the concept of polarity. Molecules are made up of atoms, and the distribution of electrons within these molecules determines their polarity.
-
Polar molecules: These molecules have an uneven distribution of charge, creating a positive and a negative end (like a magnet). Water (H₂O) is a classic example. The oxygen atom is more electronegative than the hydrogen atoms, pulling the shared electrons closer to itself and creating a slightly negative charge on the oxygen and slightly positive charges on the hydrogens. This polarity allows water molecules to form strong hydrogen bonds with each other.
-
Nonpolar molecules: These molecules have an even distribution of charge. There's no significant difference in electronegativity between the atoms, and no distinct positive or negative poles. Most oils are composed of long chains of hydrocarbons (carbon and hydrogen atoms), which are nonpolar.
The "Like Dissolves Like" Rule: This fundamental principle of chemistry dictates that polar substances tend to dissolve in polar solvents, and nonpolar substances tend to dissolve in nonpolar solvents. Water, being polar, readily dissolves other polar substances like salt and sugar. Oil, being nonpolar, dissolves other nonpolar substances like fats and grease.
Because oil and water have vastly different polarities, they are immiscible. The strong hydrogen bonds between water molecules prevent oil molecules from intermingling. The nonpolar oil molecules are repelled by the polar water molecules, leading to phase separation.
Intermolecular Forces: The Microscopic Battle
The behavior of oil and water is governed by the interplay of various intermolecular forces. These are the forces of attraction or repulsion between molecules.
-
Hydrogen bonding (in water): As mentioned earlier, water molecules are strongly attracted to each other through hydrogen bonds. This creates a cohesive network that resists the intrusion of nonpolar oil molecules.
-
London Dispersion Forces (in oil): Nonpolar molecules like those in oil experience weaker intermolecular forces called London Dispersion Forces. These forces arise from temporary fluctuations in electron distribution, creating temporary dipoles. While weaker than hydrogen bonds, these forces still contribute to the cohesion within the oil phase.
The mismatch between the strong hydrogen bonds in water and the weaker London Dispersion Forces in oil prevents them from mixing effectively. The energy required to overcome the hydrogen bonds in water and force oil molecules into the water structure is far greater than the energy gained from weak interactions between oil and water molecules.
Oil and Water: A Heterogeneous Mixture – Specifically, an Emulsion
Given the immiscibility of oil and water, their combination forms a heterogeneous mixture. More specifically, it forms an emulsion. An emulsion is a type of heterogeneous mixture where one liquid is dispersed as small droplets throughout another liquid.
In the case of oil and water, you can create a temporary emulsion by vigorously shaking the mixture. This will create tiny droplets of oil suspended in water (or vice versa). However, this emulsion is unstable. Over time, the oil and water will separate again due to the inherent differences in their densities and intermolecular forces.
Factors Affecting Emulsion Stability
While oil and water naturally separate, factors can influence the stability of an emulsion, even temporarily:
-
Emulsifiers: These substances, also known as surfactants, reduce the surface tension between oil and water, allowing the formation of smaller, more stable droplets. Soaps and detergents are common emulsifiers. They have a polar "head" and a nonpolar "tail," allowing them to interact with both water and oil, stabilizing the emulsion.
-
Temperature: Changes in temperature can affect the viscosity of both oil and water, influencing the stability of the emulsion.
-
Mixing: Vigorous mixing can create smaller droplets, leading to a temporarily more stable emulsion. However, gravity will eventually cause separation.
Beyond Oil and Water: Extending the Concepts
The principles governing the immiscibility of oil and water apply to many other liquid pairs. The "like dissolves like" rule is a powerful tool for predicting the miscibility of different substances. Understanding polarity and intermolecular forces is crucial in various fields, including:
-
Material Science: Designing new materials with specific properties often relies on controlling the interactions between different components.
-
Pharmaceuticals: Drug delivery systems often involve formulating emulsions or suspensions to enhance drug absorption and stability.
-
Food Science: Many food products, like mayonnaise and salad dressings, are emulsions stabilized by emulsifiers.
-
Environmental Science: Understanding the behavior of oil spills in water is crucial for effective cleanup efforts.
Conclusion: A Deeper Dive into Mixture Science
The seemingly simple question of what type of mixture oil and water form opens a window into the complex world of chemistry. Through the lens of polarity, intermolecular forces, and the classification of mixtures, we've uncovered a rich understanding of why these two substances don't mix and the nature of the resulting emulsion. This knowledge extends far beyond this single example, providing a foundation for understanding a wide range of phenomena in various scientific disciplines. The "like dissolves like" principle serves as a fundamental guideline in various applications, showcasing the practical implications of this seemingly simple observation about oil and water. The instability of the oil-water emulsion underscores the importance of emulsifiers in numerous industrial and everyday applications. By exploring these concepts, we gain a deeper appreciation for the intricacies of the material world around us.
Latest Posts
Latest Posts
-
The Molecular Formula For Glucose Is
Mar 30, 2025
-
Hydrogen Sulphide Gas Burns In Air To Give
Mar 30, 2025
-
Combine Terms 12a 26b 4b 16a
Mar 30, 2025
-
What Is The Difference Between Obligate And Facultative
Mar 30, 2025
-
What Is The Molecular Mass Of Ch3cooh
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Oil And Water Is What Type Of Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
