The Molecular Formula For Glucose Is
News Leon
Mar 30, 2025 · 5 min read
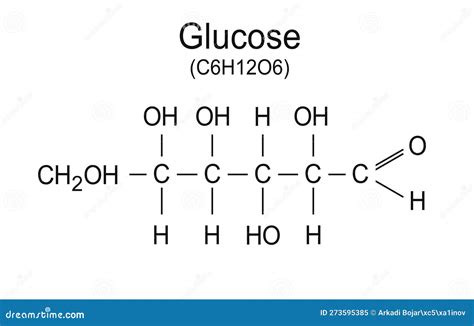
Table of Contents
The Molecular Formula for Glucose Is… C₆H₁₂O₆: A Deep Dive into the Chemistry of Sugar
The simple molecular formula, C₆H₁₂O₆, often conjures up images of sugary treats and energy boosts. But behind this concise notation lies a fascinating world of chemistry, isomerism, and biological significance. This article will delve deep into the molecular formula for glucose, exploring its structure, properties, isomers, and crucial role in biological systems. We'll also touch upon related concepts, ensuring a comprehensive understanding of this fundamental molecule.
Understanding the Basics: What C₆H₁₂O₆ Tells Us
The molecular formula, C₆H₁₂O₆, immediately reveals the elemental composition of glucose:
- 6 Carbon (C) atoms: These atoms form the backbone of the glucose molecule, creating a ring structure.
- 12 Hydrogen (H) atoms: These atoms are bonded to the carbon atoms, influencing the molecule's reactivity and shape.
- 6 Oxygen (O) atoms: These atoms are crucial for the formation of hydroxyl (-OH) groups and the overall structure's stability.
This formula, however, is insufficient to fully describe glucose. Many other sugars share the same empirical formula, highlighting the importance of understanding its structural formula and isomerism.
Delving Deeper: The Structural Formula and Isomerism of Glucose
While the molecular formula indicates the constituent atoms, it doesn't depict their arrangement. Glucose exists primarily in a cyclic form, a six-membered ring containing five carbon atoms and one oxygen atom. This ring structure is crucial for glucose's properties and reactivity. The hydroxyl groups (-OH) attached to the carbon atoms are positioned differently depending on the glucose isomer.
Glucose Isomers: A Matter of Arrangement
The same molecular formula can represent multiple distinct molecules, a phenomenon known as isomerism. Glucose is an aldohexose, meaning it's an aldehyde sugar with six carbon atoms. Several isomers exist, differing in the spatial arrangement of the hydroxyl (-OH) groups. The most important isomers are:
- α-D-Glucose: This is the most common form of glucose found in nature. The hydroxyl group on the anomeric carbon (carbon 1) is below the plane of the ring.
- β-D-Glucose: This isomer differs from α-D-glucose only in the orientation of the hydroxyl group on the anomeric carbon. It's found in cellulose, a key component of plant cell walls.
The difference in the hydroxyl group's orientation might seem minor, but it has profound implications for the molecule's biological function and reactivity. Enzymes, for example, are highly specific and only interact with particular isomers.
Other isomers of C₆H₁₂O₆ include fructose (a ketohexose) and galactose (another aldohexose). These isomers, although sharing the same molecular formula, have distinct structural arrangements and properties, leading to differences in their biological roles.
The Role of Glucose in Biological Systems: A Cornerstone of Life
Glucose is arguably the most important monosaccharide in biology. It serves as the primary source of energy for most living organisms, playing a pivotal role in metabolism.
Cellular Respiration: Glucose as Fuel
Through a series of complex biochemical reactions known as cellular respiration, glucose is broken down to release energy in the form of adenosine triphosphate (ATP). This process occurs in the mitochondria, the powerhouses of cells. The overall reaction can be simplified as:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy (ATP)
This energy is then utilized to power various cellular processes, from muscle contraction to protein synthesis.
Glucose Storage: Glycogen and Starch
Excess glucose is often stored for later use. Animals store glucose as glycogen, a highly branched polymer of glucose units. Plants, on the other hand, store glucose as starch, another glucose polymer, but with a different branching pattern. These storage forms allow organisms to access readily available energy when needed.
Structural Role: Cellulose
In plants, glucose also plays a structural role as a key component of cellulose. Cellulose is a linear polymer of glucose units linked together by β-1,4-glycosidic bonds, forming strong fibers that provide structural support to plant cell walls.
Beyond the Basics: Advanced Concepts Related to Glucose
This section delves into more complex aspects of glucose and related concepts:
Glycosidic Bonds: Linking Glucose Units
Glucose molecules can link together through glycosidic bonds, forming disaccharides (like sucrose and lactose) and polysaccharides (like starch and cellulose). The type of glycosidic bond (α or β) and the positions of linkage significantly affect the properties and function of the resulting polymer.
Glucose Metabolism: A Complex Network of Pathways
Glucose metabolism is a complex network of interconnected pathways involving numerous enzymes and regulatory molecules. These pathways regulate glucose uptake, storage, and breakdown, ensuring a balanced energy supply for the organism.
Blood Glucose Regulation: Maintaining Homeostasis
The concentration of glucose in the blood is tightly regulated by hormones like insulin and glucagon. These hormones work together to maintain blood glucose levels within a narrow range, preventing both hypoglycemia (low blood sugar) and hyperglycemia (high blood sugar).
Glucose and Health: Diabetes and Metabolic Disorders
Dysregulation of glucose metabolism can lead to serious health consequences, such as diabetes. Type 1 diabetes results from a lack of insulin production, while type 2 diabetes involves insulin resistance. Understanding glucose metabolism is crucial for diagnosing and managing these conditions.
Conclusion: The Significance of C₆H₁₂O₆
The simple molecular formula C₆H₁₂O₆ belies the incredible complexity and biological significance of glucose. From its various isomers and structural forms to its central role in cellular respiration, energy storage, and structural support, glucose is fundamental to life as we know it. Understanding its chemistry, metabolism, and regulation is crucial for advancing our knowledge of biology, medicine, and numerous other fields. Further research continues to unveil deeper insights into this ubiquitous molecule, highlighting its ongoing importance in various scientific disciplines. This molecule serves as a testament to the power of simple chemical structures to underpin the remarkable complexity of biological systems. The intricacies of isomerism, metabolic pathways, and the delicate balance of glucose regulation underscore the importance of continued research and understanding of this fundamental building block of life. From basic biochemistry to cutting-edge medical research, the exploration of glucose continues to unlock valuable knowledge and therapeutic applications.
Latest Posts
Latest Posts
-
Example Of Apology Letter For Lost Documents
Apr 01, 2025
-
A Relationship In Which Two Or More Species Benefit
Apr 01, 2025
-
Which Of The Following Is Not A Physiological Need
Apr 01, 2025
-
Greatest Amount Of Digestion Takes Place In The
Apr 01, 2025
-
All Real Numbers Are Rational Numbers True Or False
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Molecular Formula For Glucose Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
