Hydrogen Sulphide Gas Burns In Air To Give
News Leon
Mar 30, 2025 · 6 min read
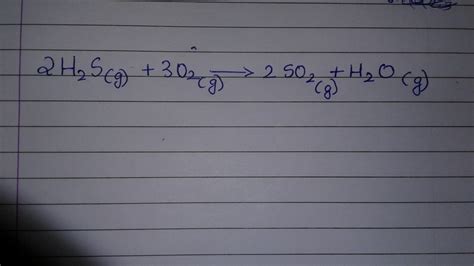
Table of Contents
Hydrogen Sulfide Gas Burns in Air to Give: A Deep Dive into the Combustion Process
Hydrogen sulfide (H₂S), a colorless gas with a characteristic foul odor resembling rotten eggs, readily reacts with oxygen (O₂) in the air to undergo combustion. This process, while seemingly simple, involves a complex interplay of chemical reactions and produces several byproducts depending on the availability of oxygen. Understanding the combustion of hydrogen sulfide is crucial in various fields, from industrial safety to environmental monitoring. This comprehensive article delves into the specifics of this reaction, exploring its chemical equation, reaction mechanisms, influencing factors, and practical applications.
The Basic Combustion Reaction
The simplest representation of hydrogen sulfide combustion is its complete combustion in an excess of oxygen. This results in the formation of sulfur dioxide (SO₂) and water (H₂O):
2H₂S(g) + 3O₂(g) → 2SO₂(g) + 2H₂O(g)
This equation illustrates the stoichiometric relationship between the reactants and products. Two moles of hydrogen sulfide react with three moles of oxygen to produce two moles of sulfur dioxide and two moles of water vapor. However, this idealized scenario rarely occurs in real-world conditions.
Incomplete Combustion and the Formation of Elemental Sulfur
When the supply of oxygen is limited, incomplete combustion occurs. This leads to the formation of elemental sulfur (S) in addition to sulfur dioxide and water. The reaction can be represented as:
2H₂S(g) + O₂(g) → 2S(s) + 2H₂O(g)
The formation of elemental sulfur is a critical aspect to consider, especially in industrial settings where hydrogen sulfide is often present in natural gas or petroleum refining processes. This elemental sulfur can deposit in pipelines and equipment, causing operational issues and safety hazards. The presence or absence of elemental sulfur is directly correlated to the oxygen-to-fuel ratio.
Reaction Mechanisms: A Deeper Look
The combustion of hydrogen sulfide isn't a single-step reaction; it's a complex process involving a series of elementary reactions. These reactions are influenced by various factors like temperature, pressure, and the presence of catalysts. While the exact mechanisms are still under investigation, several key steps are generally accepted:
Step 1: Initiation
The process begins with the initiation step, where hydrogen sulfide molecules are energized, typically through heat or a spark. This leads to the breaking of chemical bonds, creating highly reactive species like hydrogen atoms (H•) and sulfanyl radicals (HS•).
Step 2: Propagation
The propagation steps involve a chain reaction, where the reactive radicals generated in the initiation step react with oxygen molecules to produce new radicals. These radicals then continue to react with more hydrogen sulfide molecules, propagating the reaction chain. This chain reaction is responsible for the rapid and exothermic nature of hydrogen sulfide combustion.
Step 3: Branching
The reaction mechanisms can also involve branching, where a single reaction produces more than one reactive radical, accelerating the overall reaction rate. This can lead to rapid flame propagation and potential explosions if the conditions are right.
Step 4: Termination
The chain reaction eventually terminates when reactive radicals combine with each other to form stable molecules. This step slows down the combustion process.
Influencing Factors: Temperature, Pressure, and Catalysts
Several factors influence the combustion of hydrogen sulfide:
-
Temperature: The reaction rate increases significantly with temperature. A higher temperature leads to more frequent and energetic collisions between molecules, facilitating bond breakage and the formation of reactive intermediates. This is why ignition is required to initiate the combustion.
-
Pressure: Increased pressure enhances the reaction rate by increasing the concentration of reactants, leading to more frequent collisions. However, the effect of pressure on combustion is less pronounced than that of temperature.
-
Oxygen Concentration: The oxygen-to-fuel ratio is a crucial determinant. Complete combustion requires a sufficient supply of oxygen. A deficiency leads to incomplete combustion and the production of elemental sulfur.
-
Catalysts: The presence of catalysts can significantly alter the reaction rate. Certain metal oxides, for example, can act as catalysts, accelerating the combustion process by lowering the activation energy.
Environmental Implications and Safety Considerations
The combustion of hydrogen sulfide has significant environmental implications. Sulfur dioxide (SO₂), a major product of complete combustion, is a major air pollutant responsible for acid rain. It reacts with water vapor in the atmosphere to form sulfuric acid (H₂SO₄), which falls as acid rain, damaging ecosystems and infrastructure. Therefore, controlling the emissions of SO₂ from hydrogen sulfide combustion is critical for environmental protection.
Safety considerations are paramount when dealing with hydrogen sulfide. It's a highly toxic gas, even at low concentrations. Inhaling hydrogen sulfide can cause headaches, dizziness, nausea, and even death. Therefore, proper ventilation, personal protective equipment, and safety protocols are essential in any environment where hydrogen sulfide is present. Moreover, the combustion process itself can be hazardous, particularly the potential for explosions due to the flammability of hydrogen sulfide.
Industrial Applications and Utilization of Byproducts
Despite its hazardous nature, hydrogen sulfide finds application in various industrial processes. Its combustion, though requiring careful control, is used in several contexts:
-
Sulfur Recovery: In the petroleum and natural gas industries, hydrogen sulfide is often removed from sour gas streams. This removed H₂S is then burned under controlled conditions to produce elemental sulfur, a valuable byproduct used in various industries, including the production of sulfuric acid, fertilizers, and rubber. The Claus process is a widely used industrial method for this sulfur recovery.
-
Energy Production: While not a primary fuel source, hydrogen sulfide can be burned to generate energy, particularly in smaller, specialized applications. However, the environmental impact from SO₂ emissions must be mitigated through appropriate scrubbing technologies.
-
Hydrogen Production: While less common, the combustion of hydrogen sulfide can be harnessed to generate hydrogen gas through a process of steam methane reforming. However, this is generally less efficient than other methods.
Advanced Considerations and Future Research
The combustion of hydrogen sulfide continues to be an area of active research. Scientists are exploring advanced techniques to improve the efficiency of sulfur recovery, minimize SO₂ emissions, and enhance safety protocols. Research focuses on:
-
Developing more efficient catalysts: Improving catalyst design can increase the reaction rate and selectivity towards desired products, reducing unwanted byproducts like SO₂.
-
Optimizing combustion conditions: Precise control of temperature, pressure, and oxygen concentration can improve the efficiency of the process and minimize emissions.
-
Novel combustion technologies: Exploring alternative combustion technologies, such as plasma-assisted combustion, could lead to more environmentally friendly and efficient processes.
-
Advanced gas treatment technologies: Developing advanced techniques for capturing and treating SO₂ emissions is crucial for reducing the environmental impact of hydrogen sulfide combustion.
Conclusion
The combustion of hydrogen sulfide is a complex process with significant implications for safety, industrial applications, and environmental protection. Understanding the reaction mechanisms, influencing factors, and byproducts is essential for safe and responsible handling of this hazardous gas. While challenges remain, ongoing research and technological advancements offer hope for more efficient, environmentally friendly, and safe utilization of hydrogen sulfide and its combustion products. The future of H₂S combustion lies in optimizing existing technologies and developing innovative approaches that minimize environmental impact and maximize the value of the byproducts. This will ensure that this reactive and potentially hazardous gas is handled responsibly and its value harnessed while mitigating its risks.
Latest Posts
Latest Posts
-
Which Of The Following Are Found In All Viruses
Apr 01, 2025
-
The Probability Of An Impossible Event Is
Apr 01, 2025
-
In What Way Are Energy And Nutrients Similar
Apr 01, 2025
-
Example Of Apology Letter For Lost Documents
Apr 01, 2025
-
A Relationship In Which Two Or More Species Benefit
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Hydrogen Sulphide Gas Burns In Air To Give . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
