What Is The Molecular Mass Of Ch3cooh
News Leon
Mar 30, 2025 · 5 min read
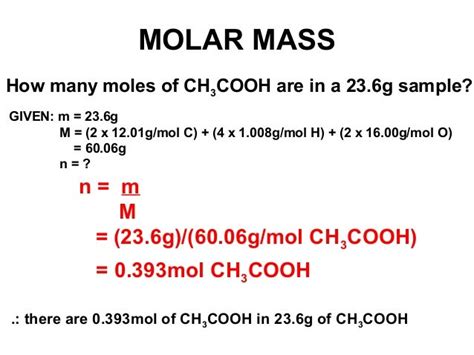
Table of Contents
What is the Molecular Mass of CH3COOH? A Deep Dive into Acetic Acid
Acetic acid, also known as ethanoic acid, is a ubiquitous organic compound with the chemical formula CH₃COOH. Its simple structure belies its importance in various fields, from biological processes to industrial applications. Understanding its molecular mass is fundamental to many chemical calculations and analyses. This article will thoroughly explore the calculation of the molecular mass of CH₃COOH, delve into its properties, and discuss its significance in different contexts.
Understanding Molecular Mass
Before we calculate the molecular mass of acetic acid, let's clarify the concept. Molecular mass (also known as molecular weight) represents the mass of a molecule, expressed in atomic mass units (amu) or Daltons (Da). It's essentially the sum of the atomic masses of all the atoms constituting the molecule. This value is crucial in stoichiometry, determining concentrations, and understanding chemical reactions.
Calculating the Molecular Mass of CH3COOH
To calculate the molecular mass of CH₃COOH, we need the atomic masses of its constituent elements: carbon (C), hydrogen (H), and oxygen (O). Standard atomic masses are typically used, which are weighted averages reflecting the isotopic composition of the elements in nature. These values are readily available in the periodic table.
- Carbon (C): Approximately 12.01 amu
- Hydrogen (H): Approximately 1.01 amu
- Oxygen (O): Approximately 16.00 amu
Now, let's break down the CH₃COOH molecule:
- 2 Carbon atoms (C): 2 * 12.01 amu = 24.02 amu
- 4 Hydrogen atoms (H): 4 * 1.01 amu = 4.04 amu
- 2 Oxygen atoms (O): 2 * 16.00 amu = 32.00 amu
Total Molecular Mass: 24.02 amu + 4.04 amu + 32.00 amu = 60.06 amu
Therefore, the molecular mass of CH₃COOH is approximately 60.06 amu or 60.06 g/mol. The g/mol unit is used when dealing with molar mass, which represents the mass of one mole of the substance (6.022 x 10²³ molecules). In essence, 60.06 g of acetic acid contains one mole of acetic acid molecules.
The Significance of Acetic Acid's Molecular Mass
Knowing the molecular mass of acetic acid is crucial in several applications:
-
Stoichiometric Calculations: In chemical reactions involving acetic acid, its molecular mass is essential for calculating the amounts of reactants and products. For instance, in a titration, knowing the molecular mass allows for precise determination of the concentration of an unknown solution.
-
Solution Preparation: Preparing solutions of a specific concentration requires precise weighing of the solute. The molecular mass of acetic acid is necessary to determine the mass required to create a solution with a desired molarity. For example, preparing a 1M solution of acetic acid requires dissolving 60.06 g of acetic acid in 1 liter of solvent.
-
Spectroscopic Analysis: Techniques like mass spectrometry directly measure the mass-to-charge ratio of ions. The molecular mass of acetic acid helps identify the compound and understand its fragmentation patterns in mass spectra.
-
Physicochemical Property Prediction: Molecular mass is a key parameter in predicting various physicochemical properties of acetic acid, such as boiling point, melting point, and solubility. These properties are essential for various applications, including industrial processes and biological studies.
-
Pharmaceutical and Biochemical Applications: Acetic acid is used as a reagent in numerous chemical synthesis routes in the pharmaceutical industry for creating active ingredients or intermediates in drug production. Its molecular mass is vital for accurate scaling up of production processes, ensuring precise dosages and purity.
Properties and Applications of Acetic Acid
Acetic acid exhibits several characteristic properties that contribute to its widespread applications. It's a weak acid, meaning it only partially dissociates in water, releasing hydrogen ions (H⁺). This weak acidity makes it suitable for various applications, including food preservation and as a component in household cleaners.
Key Properties of Acetic Acid:
- Weak Acidity (pKa ~ 4.76): It partially dissociates in water, contributing to its acidic nature.
- Water Solubility: Completely miscible with water, allowing for easy solution preparation.
- Sharp, Pungent Odor: Its characteristic vinegar smell is readily recognizable.
- Colorless Liquid: Pure acetic acid is a colorless liquid at room temperature.
- Boiling Point (118.1 °C): Relatively high boiling point compared to its molecular size.
Applications of Acetic Acid:
The diverse applications of acetic acid stem from its properties. Its most common form is vinegar, a dilute solution of acetic acid (typically 4-8%). Beyond vinegar, acetic acid finds uses in:
- Food Industry: Preservative, flavoring agent, and acidity regulator.
- Industrial Processes: Production of plastics, textiles, and other chemicals.
- Medical Applications: Antiseptic, treatment for warts, and component in various pharmaceutical formulations.
- Household Cleaning Products: Many household cleaners utilize acetic acid for its cleaning and disinfecting properties.
Isotopic Variations and Molecular Mass
While the standard atomic masses were used in our calculation, it's important to note that isotopes exist. Isotopes are atoms of the same element with differing numbers of neutrons, resulting in slightly different atomic masses. This can affect the overall molecular mass of acetic acid, though the variation is typically small. Highly precise measurements might require considering isotopic abundances for more accurate calculations.
Conclusion
The molecular mass of acetic acid (CH₃COOH) is approximately 60.06 amu or 60.06 g/mol. This value is fundamental in various chemical calculations, applications, and analytical techniques. Understanding its molecular mass allows for accurate stoichiometric calculations, solution preparation, and predictions of physicochemical properties. The versatile nature of acetic acid, ranging from everyday use in vinegar to significant roles in industrial processes and the pharmaceutical industry, highlights the importance of this simple yet crucial organic compound. Further exploration of its properties and applications reveals its significance across various disciplines. The accuracy of molecular mass calculations plays a vital role in ensuring the precision and efficacy of many scientific and industrial processes.
Latest Posts
Latest Posts
-
Greatest Amount Of Digestion Takes Place In The
Apr 01, 2025
-
All Real Numbers Are Rational Numbers True Or False
Apr 01, 2025
-
Which Of The Following Is A Function That Money Serves
Apr 01, 2025
-
The Most Abundant Compound In Most Living Things Is
Apr 01, 2025
-
How Can We Change The Polarity Of An Electromagnet
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Mass Of Ch3cooh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
