Number Of Valence Electrons Of Copper
News Leon
Mar 25, 2025 · 5 min read
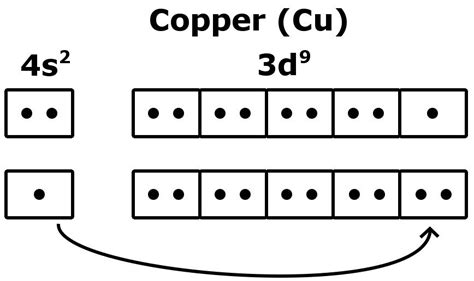
Table of Contents
Unveiling the Mystery: The Number of Valence Electrons in Copper
Copper, a reddish-orange metal renowned for its excellent conductivity and malleability, plays a crucial role in various industries, from electrical wiring to plumbing. Understanding its electronic structure, specifically the number of valence electrons, is key to comprehending its unique properties and diverse applications. This comprehensive article delves into the intricacies of copper's valence electrons, exploring its electronic configuration, oxidation states, and the implications for its chemical behavior.
Understanding Valence Electrons: The Foundation
Before diving into the specifics of copper, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, dictating an element's reactivity and the types of bonds it can form. The number of valence electrons directly influences an element's chemical properties and its position within the periodic table.
Determining Valence Electrons: A Simple Approach
For many elements, determining the number of valence electrons is relatively straightforward. The group number (using the American system) in the periodic table directly corresponds to the number of valence electrons for main group elements (groups 1-18). However, this simplification doesn't always apply to transition metals like copper, which occupy the d-block of the periodic table.
The Electronic Configuration of Copper: A Deeper Dive
To accurately determine the number of valence electrons in copper, we need to examine its electronic configuration. Copper (Cu) has an atomic number of 29, indicating 29 protons and 29 electrons in a neutral atom. The electronic configuration of copper is typically written as:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰
This configuration might seem counterintuitive. You might expect a 4s² 3d⁹ configuration. However, a completely filled 3d subshell and a half-filled 4s subshell provide greater stability due to exchange energy and other quantum mechanical effects. This slight deviation from the expected filling order highlights the complexities of electron configurations in transition metals.
The Valence Electrons of Copper: The Complicated Truth
Now, the crucial question: how many valence electrons does copper have? This is where things get slightly more nuanced than simply looking at the highest principal quantum number (n=4). While the 4s electron is undoubtedly a valence electron, the involvement of the 3d electrons in chemical bonding is context-dependent.
The Role of d-Electrons in Bonding
Unlike main group elements, transition metals like copper can utilize both their s and d electrons in chemical bonding. This is because the energy difference between the (n-1)d and ns orbitals is relatively small. Therefore, the number of valence electrons in copper isn't a fixed number; it can vary depending on the specific chemical context.
Oxidation States and Valence Electrons
Copper exhibits variable oxidation states, commonly +1 (cuprous) and +2 (cupric). These oxidation states reflect the number of electrons lost by copper in forming chemical bonds.
-
Cu⁺ (Cuprous): In the +1 oxidation state, copper loses one electron, typically the 4s electron. This leaves the 3d shell completely filled. In this case, we can consider copper to have one valence electron.
-
Cu²⁺ (Cupric): In the +2 oxidation state, copper loses two electrons. This usually involves the loss of the 4s electron and one 3d electron. Here, we could consider copper to have two valence electrons.
It's important to note that the description of losing a 3d electron is a simplification. The reality is more complex, involving the hybridization of orbitals and the delocalization of electrons in the metal structure.
Implications of Variable Valence Electrons: Chemical Behavior
The variable valence electron behavior of copper significantly impacts its chemical properties. This explains its ability to form various compounds with different oxidation states and its diverse reactivity.
Copper's Unique Reactivity
Copper's capacity to exhibit both +1 and +2 oxidation states allows it to participate in a wide range of redox reactions. It can act as both an oxidizing agent (accepting electrons) and a reducing agent (donating electrons), depending on the reaction environment.
Examples of Copper Compounds and Oxidation States
Several common copper compounds illustrate its variable oxidation states:
- Copper(I) oxide (Cu₂O): Copper is in the +1 oxidation state.
- Copper(II) oxide (CuO): Copper is in the +2 oxidation state.
- Copper(I) chloride (CuCl): Copper is in the +1 oxidation state.
- Copper(II) chloride (CuCl₂): Copper is in the +2 oxidation state.
- Copper sulfate pentahydrate (CuSO₄·5H₂O): Copper is in the +2 oxidation state.
These examples demonstrate the versatility of copper in forming compounds with different stoichiometries and properties based on its variable valence electron involvement.
Copper's Applications: A Consequence of its Electronic Structure
The unique electronic structure and variable valence of copper are directly responsible for its widespread applications.
Electrical Conductivity: A Hallmark Property
Copper's high electrical conductivity is a direct consequence of its electronic configuration. The loosely held valence electrons are easily mobile, allowing for efficient current flow. This makes copper an indispensable material in electrical wiring and various electronic components.
Thermal Conductivity: Another Key Attribute
Copper's excellent thermal conductivity, also linked to its electronic structure, makes it ideal for heat exchangers, heat sinks, and other applications requiring efficient heat transfer.
Malleability and Ductility: Shaping Copper
Copper's malleability and ductility, its ability to be easily shaped and drawn into wires, are related to the metallic bonding arising from its valence electrons. These properties facilitate the manufacturing of various copper products.
Conclusion: Beyond a Simple Answer
Determining the precise number of valence electrons in copper isn't a straightforward task. While the simplest answer might point to one (from the 4s orbital), the involvement of d-electrons in chemical bonding necessitates a more nuanced understanding. The variable valence of copper, reflecting its ability to utilize both s and d electrons, dictates its diverse chemical behavior and wide-ranging applications. This understanding highlights the complexity and richness of transition metal chemistry and the importance of considering the context when discussing the valence electrons of these elements. The ability of copper to shift between oxidation states, impacting its conductivity, reactivity, and malleability, cements its importance in numerous technological applications. Further research into the intricacies of copper's electronic structure will undoubtedly lead to even more innovative applications in the future.
Latest Posts
Latest Posts
-
In An Atom The Nucleus Contains
Mar 28, 2025
-
What Does Chr Do In Python
Mar 28, 2025
-
Whats Half Of 1 1 2 Tsp
Mar 28, 2025
-
Genes Had Been Absent On The Chromosomes
Mar 28, 2025
-
Which Of The Following Is A Nonrenewable Source Of Energy
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons Of Copper . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
