In An Atom The Nucleus Contains
News Leon
Mar 28, 2025 · 7 min read
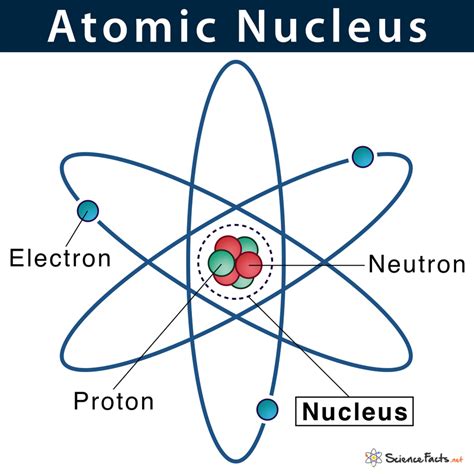
Table of Contents
In an Atom, the Nucleus Contains: A Deep Dive into the Heart of Matter
The atom, the fundamental building block of matter, is a fascinating microcosm of energy and structure. While often depicted as a simple planetary model with electrons orbiting a central nucleus, the reality is far more complex and intriguing. This article delves deep into the heart of the atom, exploring the composition, properties, and significance of the atomic nucleus.
What is the Atomic Nucleus?
The atomic nucleus is the dense central region of an atom, containing protons and neutrons, collectively known as nucleons. It's incredibly small, occupying only a tiny fraction of the atom's overall volume, yet it accounts for virtually all of the atom's mass. The nucleus is held together by the strong nuclear force, one of the four fundamental forces of nature, which overcomes the electrostatic repulsion between the positively charged protons.
Size and Density
The nucleus's size is typically measured in femtometers (fm), where 1 fm = 10<sup>-15</sup> meters. To put this into perspective, if an atom were the size of a football stadium, the nucleus would be about the size of a pea at the center. Despite its minuscule size, the nucleus possesses an astonishingly high density. Its density is on the order of 10<sup>17</sup> kg/m³, which is trillions of times denser than the densest materials found on Earth.
Composition of the Nucleus: Protons and Neutrons
The nucleus is composed of two types of nucleons:
Protons: The Positively Charged Particles
Protons are positively charged particles with a charge of +1e, where 'e' represents the elementary charge (approximately 1.602 x 10<sup>-19</sup> Coulombs). The number of protons in an atom's nucleus defines the atom's atomic number, which determines its chemical identity. For example, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and so on. The arrangement of protons in the nucleus significantly impacts the atom's properties and behavior.
Neutrons: The Neutral Particles
Neutrons are electrically neutral particles with a mass slightly larger than that of a proton. While they don't contribute to the atom's charge, they play a crucial role in nuclear stability. The number of neutrons in an atom's nucleus is known as the neutron number. The sum of the proton number and the neutron number gives the mass number of the atom.
Isotopes: Variations in Neutron Number
Atoms of the same element can have different numbers of neutrons, resulting in isotopes. Isotopes of an element have the same atomic number (same number of protons) but different mass numbers (different numbers of neutrons). Some isotopes are stable, while others are radioactive, meaning they undergo radioactive decay, transforming into different atoms over time. This radioactive decay is a crucial aspect of nuclear physics and has numerous applications in medicine, industry, and research.
Nuclear Forces: What Holds the Nucleus Together?
The incredibly strong and short-range strong nuclear force is responsible for binding protons and neutrons together within the nucleus. This force is much stronger than the electromagnetic force, which would otherwise cause the positively charged protons to repel each other and cause the nucleus to fly apart. The strong nuclear force acts over extremely short distances, only within the confines of the nucleus itself.
The Role of the Strong Nuclear Force in Stability
The balance between the strong nuclear force and the electromagnetic force is critical to nuclear stability. In stable nuclei, the strong force is strong enough to overcome the electrostatic repulsion between protons, holding the nucleus together. However, as the number of protons increases, the electromagnetic repulsion becomes increasingly significant, making it harder for the strong force to maintain stability. This is why elements with very high atomic numbers tend to be radioactive and less stable.
Nuclear Models: Understanding Nuclear Structure
Several models have been developed to describe the structure and behavior of the atomic nucleus. These models offer different perspectives on the complex interactions within the nucleus.
The Liquid Drop Model
The liquid drop model treats the nucleus as a drop of incompressible liquid, where nucleons interact with their immediate neighbors. This model successfully explains several aspects of nuclear behavior, including nuclear fission, the process by which a heavy nucleus splits into two lighter nuclei.
The Shell Model
The shell model considers the nucleons to be arranged in energy levels or shells, similar to the arrangement of electrons in an atom. This model explains the unusual stability of certain nuclei with specific numbers of protons or neutrons, known as magic numbers. These magic numbers (2, 8, 20, 28, 50, 82, and 126) correspond to closed shells in the nuclear structure. Nuclei with magic numbers of protons and/or neutrons are exceptionally stable and less prone to radioactive decay.
The Collective Model
The collective model combines features of both the liquid drop model and the shell model. It accounts for both the collective motion of nucleons and the individual particle behavior within the nucleus. This model provides a more nuanced understanding of nuclear structure and properties.
Nuclear Reactions: Transformations within the Nucleus
Nuclear reactions involve changes in the composition of the atomic nucleus. These reactions can release tremendous amounts of energy, far exceeding the energy released in chemical reactions.
Nuclear Fission
Nuclear fission is the splitting of a heavy nucleus into two lighter nuclei, accompanied by the release of a large amount of energy and neutrons. This process is the basis of nuclear power plants and nuclear weapons. The chain reaction of fission is a remarkable demonstration of the power locked within the nucleus.
Nuclear Fusion
Nuclear fusion is the process by which two light nuclei combine to form a heavier nucleus, also releasing a significant amount of energy. Fusion is the energy source of the sun and other stars, where hydrogen nuclei fuse to form helium, releasing vast amounts of energy. Harnessing controlled fusion on Earth is a major research goal, with the potential to provide a clean and virtually inexhaustible energy source.
Applications of Nuclear Physics: The Impact on Society
Our understanding of the atomic nucleus has led to significant technological advancements and applications across various fields:
Nuclear Medicine
Radioactive isotopes are widely used in medical imaging and treatment. Techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography) utilize radioactive tracers to visualize organs and tissues, aiding in diagnosis. Radiotherapy uses radiation to target and destroy cancerous cells.
Nuclear Power
Nuclear power plants generate electricity by harnessing the energy released from nuclear fission. While controversial, nuclear power provides a low-carbon source of energy and contributes significantly to the energy needs of several countries.
Industrial Applications
Radioactive isotopes find various applications in industry, including gauging thickness, tracing materials, and sterilization. Nuclear techniques are also used in archaeology and geology for dating artifacts and rocks.
Conclusion: The Nucleus – A Realm of Unparalleled Power and Complexity
The atomic nucleus, despite its incredibly small size, is a realm of unparalleled power and complexity. Its composition, the forces governing its structure, and the reactions it undergoes have profound implications for our understanding of matter and energy. The ongoing research and technological advancements in nuclear physics continue to unlock the secrets of this fascinating subatomic world and shape the future of energy, medicine, and technology. The nucleus remains a source of intense study and offers many more revelations yet to be discovered. Further research into the intricacies of nuclear forces and behavior promises to unveil even deeper insights into the fundamental laws of nature and opens up opportunities for innovative applications in the years to come. The journey into the heart of matter continues, with the nucleus as the central focus of ongoing scientific exploration and innovation.
Latest Posts
Latest Posts
-
A Perfectly Elastic Demand Curve Implies That The Firm
Mar 31, 2025
-
Which Of The Following Are Complementary Bases In Dna
Mar 31, 2025
-
Classify The Following As A Homogeneous Or A Heterogeneous Mixture
Mar 31, 2025
-
What Physical Quantity Does The Slope Represent
Mar 31, 2025
-
An Object Becomes Positively Charged By Gaining Protons
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about In An Atom The Nucleus Contains . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
