Net Ionic Equation For Naoh Hcl
News Leon
Mar 25, 2025 · 5 min read
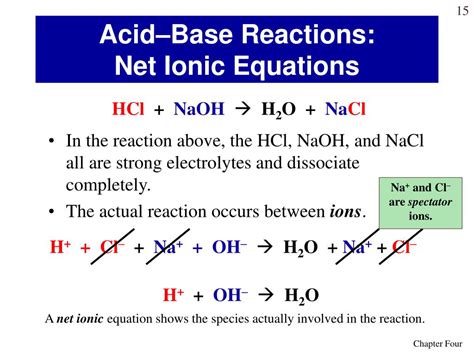
Table of Contents
Net Ionic Equation for NaOH + HCl: A Deep Dive into Acid-Base Reactions
The reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) is a classic example of a strong acid-strong base neutralization reaction. Understanding this reaction, particularly its net ionic equation, is fundamental to grasping the concepts of acid-base chemistry and ionic solutions. This article will delve into the intricacies of this reaction, explaining the complete ionic equation, the net ionic equation, and the significance of spectator ions. We'll also explore the applications and implications of this reaction in various contexts.
Understanding the Reactants: NaOH and HCl
Before diving into the equation, let's briefly review the properties of the reactants:
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as lye or caustic soda, is a strong base. This means it completely dissociates in water, releasing sodium ions (Na⁺) and hydroxide ions (OH⁻). Its strong basicity stems from the hydroxide ion's ability to readily accept protons (H⁺). NaOH is widely used in various industrial processes, including soap making, paper production, and drain cleaning. Its corrosive nature necessitates careful handling.
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid, meaning it completely dissociates in water into hydrogen ions (H⁺) and chloride ions (Cl⁻). The hydrogen ions, which are essentially protons, are responsible for HCl's acidic properties. It's a crucial reagent in many chemical processes, including industrial cleaning, metal refining, and the production of various chemicals. HCl, like NaOH, is corrosive and requires careful handling.
The Complete Ionic Equation: Showing All Ions
When NaOH and HCl react in aqueous solution, they undergo a neutralization reaction, producing water and salt. The complete ionic equation displays all the ions present in the solution, both before and after the reaction. This provides a detailed picture of the ionic species involved.
The complete ionic equation for the reaction between NaOH and HCl is:
Na⁺(aq) + OH⁻(aq) + H⁺(aq) + Cl⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
In this equation:
Na⁺(aq),OH⁻(aq),H⁺(aq), andCl⁻(aq)represent the aqueous ions of sodium, hydroxide, hydrogen, and chloride, respectively. The "(aq)" indicates that these ions are dissolved in water.H₂O(l)represents liquid water.
The Net Ionic Equation: Focusing on the Essential Reaction
The net ionic equation simplifies the complete ionic equation by removing the spectator ions. Spectator ions are ions that appear on both sides of the complete ionic equation, meaning they do not participate directly in the reaction. In this case, Na⁺ and Cl⁻ are spectator ions. The net ionic equation only shows the species that actually react.
The net ionic equation for the NaOH and HCl reaction is:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation clearly shows the essence of the neutralization reaction: a proton (H⁺) from the acid reacts with a hydroxide ion (OH⁻) from the base to form water. This is the core chemical change occurring.
Significance of Spectator Ions
While spectator ions don't directly participate in the main reaction, they're still crucial parts of the solution. They contribute to the overall ionic strength and properties of the solution. Understanding spectator ions helps in simplifying complex reactions and focusing on the essential chemical processes.
Applications and Implications
The neutralization reaction between NaOH and HCl has numerous practical applications:
Titrations
This reaction is fundamental to acid-base titrations. Titrations are analytical techniques used to determine the concentration of an unknown solution using a solution of known concentration. By carefully measuring the volume of HCl needed to neutralize a known volume of NaOH (or vice-versa), the concentration of the unknown solution can be precisely calculated. This technique is widely used in chemistry, biochemistry, and environmental science.
pH Control
In many industrial processes and chemical experiments, precise pH control is crucial. The reaction between NaOH and HCl can be used to adjust the pH of a solution by carefully adding either acid or base to reach the desired pH level. This is essential in processes like wastewater treatment and chemical synthesis.
Chemical Synthesis
This reaction is often used as a step in various chemical syntheses where a neutral pH is required for a specific reaction to proceed efficiently or to avoid undesirable side reactions. Many chemical reactions are sensitive to pH changes, and this neutralization reaction plays a key role in creating the desired reaction environment.
Variations and Considerations
While the reaction between NaOH and HCl is straightforward, variations can occur based on factors like:
Concentrations
The concentrations of NaOH and HCl significantly impact the reaction's rate and the heat generated during neutralization. Higher concentrations lead to faster reactions and more significant heat release.
Temperature
Temperature affects the rate of reaction. Higher temperatures generally result in faster reaction rates.
Presence of Other Ions
The presence of other ions in the solution can affect the activity coefficients of the reacting ions, influencing the overall reaction rate and equilibrium.
Non-Ideal Behavior
At very high concentrations, deviations from ideal behavior can occur, leading to slight variations in the observed reaction behavior compared to the theoretical predictions based on the net ionic equation.
Conclusion: The Importance of the Net Ionic Equation
The net ionic equation for the NaOH + HCl reaction, H⁺(aq) + OH⁻(aq) → H₂O(l), concisely describes the fundamental chemical process of neutralization. Understanding this equation is critical for comprehending acid-base chemistry, performing titrations, controlling pH levels, and interpreting many chemical reactions in various applications. This seemingly simple reaction is a cornerstone of chemistry, underpinning a wide array of practical applications in diverse fields. The ability to write and interpret both complete and net ionic equations is an essential skill for any student or professional working with aqueous solutions and chemical reactions. The exploration of spectator ions and their significance further enriches our understanding of the dynamics of ionic solutions and their impact on reaction pathways. By mastering these concepts, we gain a deeper appreciation for the elegance and power of chemical principles. This foundational knowledge serves as a springboard for exploring more complex chemical phenomena and expanding our capabilities in chemical analysis and synthesis.
Latest Posts
Latest Posts
-
The Fibrous Connective Tissue That Wraps Muscle Is Called
Mar 26, 2025
-
An Oscillator Consists Of A Block Attached To A Spring
Mar 26, 2025
-
Ecg Is A Graphic Recording Of
Mar 26, 2025
-
The Most Abundant Type Of Immunoglobulin Is
Mar 26, 2025
-
What Does The Slope Of A Distance Time Graph Represent
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation For Naoh Hcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
