Name The Following Molecule By Its Iupac Name
News Leon
Mar 23, 2025 · 6 min read
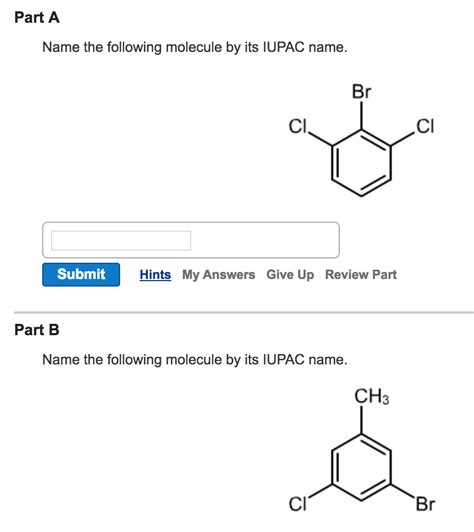
Table of Contents
Naming Organic Molecules: A Comprehensive Guide to IUPAC Nomenclature
Organic chemistry can feel daunting, particularly when it comes to naming complex molecules. But with a systematic approach, mastering IUPAC (International Union of Pure and Applied Chemistry) nomenclature becomes achievable. This comprehensive guide will walk you through the process, equipping you with the skills to confidently name a wide variety of organic molecules. We'll cover alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and more, providing numerous examples along the way.
Understanding the Fundamentals of IUPAC Nomenclature
Before diving into specific examples, let's establish the core principles of IUPAC nomenclature. The system relies on a set of rules to ensure a unique and unambiguous name for every organic molecule. These rules prioritize:
- Identifying the Parent Chain: This is the longest continuous carbon chain within the molecule. This chain forms the basis of the molecule's name.
- Numbering the Parent Chain: The parent chain is numbered to assign the lowest possible numbers to substituents (groups attached to the main chain).
- Identifying and Naming Substituents: These are the groups attached to the parent chain. They are named systematically and their positions are indicated by the numbers assigned to the parent chain.
- Combining the Information: The complete name integrates the substituent names, their positions, and the parent chain name.
Naming Alkanes: The Foundation of Organic Nomenclature
Alkanes are saturated hydrocarbons (containing only single bonds). Their names form the foundation for naming many other organic molecules.
- Meth- (1 carbon): Methane (CH₄)
- Eth- (2 carbons): Ethane (CH₃CH₃)
- Prop- (3 carbons): Propane (CH₃CH₂CH₃)
- But- (4 carbons): Butane (CH₃CH₂CH₂CH₃)
- Pent- (5 carbons): Pentane (CH₃CH₂CH₂CH₂CH₃)
- Hex- (6 carbons): Hexane (CH₃CH₂CH₂CH₂CH₂CH₃)
- Hept- (7 carbons): Heptane (CH₃(CH₂)₅CH₃)
- Oct- (8 carbons): Octane (CH₃(CH₂)₆CH₃)
- Non- (9 carbons): Nonane (CH₃(CH₂)₇CH₃)
- Dec- (10 carbons): Decane (CH₃(CH₂)₈CH₃)
Beyond ten carbons, Greek prefixes are used (undecane, dodecane, tridecane, etc.).
Branched Alkanes: Incorporating Substituents
When alkanes have branches (alkyl groups), the naming process becomes slightly more complex.
- Identify the longest continuous carbon chain: This becomes the parent alkane.
- Number the parent chain: Start numbering from the end that gives the substituents the lowest possible numbers.
- Name the substituents: Alkyl groups are named by replacing the "-ane" ending of the alkane with "-yl" (e.g., methyl, ethyl, propyl).
- Combine the information: List the substituents alphabetically, preceding each with its position number. If multiple substituents of the same type are present, use prefixes like di-, tri-, tetra-, etc., and indicate their positions.
Example: Consider the molecule CH₃CH(CH₃)CH₂CH₃
- Longest chain: 4 carbons (butane)
- Numbering: Number from the end closest to the methyl group.
- Substituents: One methyl group on carbon 2.
- Name: 2-Methylbutane
More Complex Examples of Alkane Nomenclature:
- 2,3-Dimethylpentane: CH₃CH(CH₃)CH(CH₃)CH₂CH₃
- 3-Ethyl-2,4-dimethylhexane: CH₃CH(CH₃)CH(C₂H₅)CH(CH₃)CH₂CH₃
- 2,2,4-Trimethylpentane (Isooctane): CH₃C(CH₃)₂CH₂CH(CH₃)CH₃
Alkenes and Alkynes: Incorporating Unsaturation
Alkenes contain carbon-carbon double bonds, and alkynes contain carbon-carbon triple bonds. Their naming follows similar principles to alkanes, but with additional considerations:
- Identify the longest chain containing the double or triple bond.
- Number the chain to give the double or triple bond the lowest possible number. The position of the double or triple bond is indicated by the number of the first carbon atom involved in the multiple bond.
- Use the suffix "-ene" for alkenes and "-yne" for alkynes.
- Combine the information as before, prioritizing the location of the multiple bond.
Examples:
- 1-Butene: CH₂=CHCH₂CH₃
- 2-Pentene: CH₃CH=CHCH₂CH₃
- 2-Methyl-2-butene: CH₃C(CH₃)=CHCH₃
- 1-Hexyne: CH≡C(CH₂)₃CH₃
Cycloalkanes: Handling Cyclic Structures
Cycloalkanes are saturated hydrocarbons forming a ring structure. Their names begin with "cyclo-" followed by the alkane name corresponding to the number of carbons in the ring.
- Cyclopropane: A three-carbon ring.
- Cyclobutane: A four-carbon ring.
- Cyclopentane: A five-carbon ring.
- Cyclohexane: A six-carbon ring.
Substituents on cycloalkanes are numbered to give the lowest possible numbers, often starting from the substituent with alphabetical priority.
Functional Groups: Adding Complexity to Nomenclature
Beyond alkanes, alkenes, and alkynes, organic chemistry encompasses numerous functional groups – atoms or groups of atoms with characteristic chemical properties. These groups are incorporated into the IUPAC name using specific prefixes or suffixes.
Alcohols (-OH): The suffix "-ol" is added to the parent alkane name, and the position of the hydroxyl group (-OH) is indicated by a number.
- Ethanol: CH₃CH₂OH
- Propan-1-ol: CH₃CH₂CH₂OH
- Propan-2-ol: CH₃CH(OH)CH₃
Aldehydes (-CHO): The suffix "-al" is used, and the aldehyde group is always at the end of the chain, so no number is needed.
- Ethanal: CH₃CHO
- Propanal: CH₃CH₂CHO
Ketones (C=O): The suffix "-one" is used, and the position of the carbonyl group (C=O) is indicated by a number.
- Propan-2-one (Acetone): CH₃COCH₃
- Butan-2-one: CH₃COCH₂CH₃
Carboxylic Acids (-COOH): The suffix "-oic acid" is used. The carboxylic acid group is always at the end of the chain, so no number is needed.
- Ethanoic acid (Acetic acid): CH₃COOH
- Propanoic acid: CH₃CH₂COOH
Amines (-NH₂): The prefix "amino-" is used to indicate the presence of an amino group.
- Aminomethane (Methylamine): CH₃NH₂
- Aminoethane (Ethylamine): CH₃CH₂NH₂
Ethers (R-O-R'): Ethers are named by listing the alkyl groups alphabetically followed by "ether".
- Methoxyethane: CH₃OCH₂CH₃
- Ethoxyethane (Diethyl ether): CH₃CH₂OCH₂CH₃
Halogens (F, Cl, Br, I): Halogen substituents are named fluoro-, chloro-, bromo-, and iodo-, respectively.
- 1-Chloropropane: CH₃CH₂CH₂Cl
- 2-Bromobutane: CH₃CHBrCH₂CH₃
Putting it All Together: Naming Complex Molecules
Many organic molecules contain multiple functional groups and substituents. In such cases, a hierarchical approach is essential:
- Identify the principal functional group: This is the functional group that determines the suffix of the name (e.g., carboxylic acid > aldehyde > ketone > alcohol).
- Identify the parent chain: This is the longest continuous carbon chain containing the principal functional group.
- Number the parent chain: Prioritize the position of the principal functional group and then other substituents.
- Name the substituents: List the substituents alphabetically, including their positions.
- Combine the information: The complete name integrates the substituent names, their positions, and the parent chain name with the appropriate suffix for the principal functional group.
Example: Consider the molecule CH₃CH(OH)CH₂COOH
- Principal functional group: Carboxylic acid (-COOH)
- Parent chain: 3 carbons (propane)
- Numbering: The carboxylic acid group is at position 1, and the hydroxyl group is at position 2.
- Substituents: 2-hydroxy
- Name: 2-Hydroxypropanoic acid (or Lactic Acid)
This comprehensive guide provides a strong foundation for mastering IUPAC nomenclature. Remember that practice is key. The more molecules you name, the more comfortable and confident you will become in applying these rules. Refer to authoritative organic chemistry textbooks and online resources for further examples and detailed explanations. While this guide covers many common functional groups, exploring specialized nomenclature for more complex molecules will deepen your understanding. Consistent practice and a systematic approach will undoubtedly enhance your ability to navigate the intricate world of organic molecule naming.
Latest Posts
Latest Posts
-
Do Birds Have 4 Chambered Heart
Mar 25, 2025
-
One Atomic Mass Unit Is Equal To
Mar 25, 2025
-
Acetic Acid Reacts With Sodium Hydroxide
Mar 25, 2025
-
Two Opposite Charges Separated By A Small Distance
Mar 25, 2025
-
How Many Light Years To The Moon
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Name The Following Molecule By Its Iupac Name . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
