Two Opposite Charges Separated By A Small Distance
News Leon
Mar 25, 2025 · 6 min read
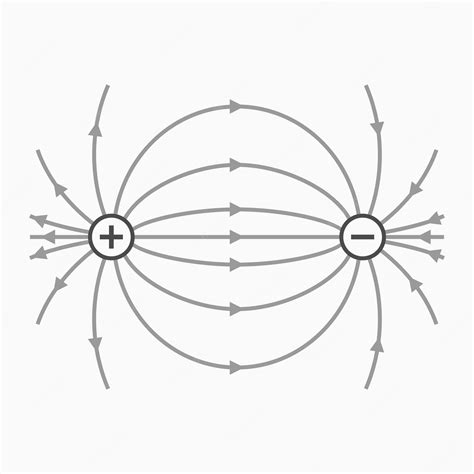
Table of Contents
Two Opposite Charges Separated by a Small Distance: A Deep Dive into Dipoles
The concept of two opposite charges separated by a small distance is fundamental to electromagnetism and has far-reaching implications across various scientific fields. This arrangement, known as a dipole, is surprisingly ubiquitous, impacting everything from the behavior of molecules to the design of advanced technologies. This comprehensive article will delve into the physics of dipoles, exploring their properties, applications, and significance in understanding the electromagnetic world.
Understanding the Dipole: A Foundation in Electrostatics
At its core, a dipole consists of two point charges of equal magnitude but opposite sign (+q and -q) separated by a small distance, denoted as 'd'. This seemingly simple configuration generates a complex and fascinating electric field. The electric dipole moment, a vector quantity denoted by p, is crucial in characterizing the dipole. It's defined as:
p = qd
where the direction of p points from the negative charge to the positive charge. The magnitude of the dipole moment, |p|, represents the strength of the dipole. The smaller the distance 'd', the more compact and potent the dipole. It's important to note that the dipole moment is independent of the coordinate system used. This invariant property simplifies calculations and analysis significantly.
Electric Field of a Dipole: Far-Field and Near-Field Approximations
The electric field generated by a dipole is complex, varying significantly depending on the distance from the dipole. We typically analyze the field in two approximations:
1. Far-field approximation: At distances significantly larger than the separation 'd' (r >> d), the electric field can be approximated as a dipole field. The field lines emanate from the positive charge and terminate at the negative charge, exhibiting a characteristic pattern. The electric field strength (E) at a distance 'r' from the center of the dipole, along the axis of the dipole, is given by:
E = (1/(4πε₀)) * (2p/r³)
where ε₀ is the permittivity of free space. Notice the inverse cube dependence on the distance. This implies that the field strength decreases rapidly with distance.
2. Near-field approximation: Close to the dipole (r ≤ d), the field is dominated by the individual contributions of the charges. The simple dipole approximation breaks down, and a more complex calculation is required to determine the electric field precisely. This region reveals a more intricate field pattern, exhibiting the individual effects of the two charges. Numerical methods are often employed for accurate calculations in the near-field region.
Dipole Moments in Molecules: A Microscopic Perspective
The concept of dipoles extends beyond the realm of point charges. Molecules, composed of atoms with differing electronegativities, often exhibit inherent dipole moments. Electronegativity refers to an atom's ability to attract electrons in a chemical bond. When atoms with different electronegativities bond, the electron distribution becomes uneven, leading to a separation of charge and the formation of a molecular dipole.
For instance, in a water molecule (H₂O), the oxygen atom is significantly more electronegative than the hydrogen atoms. This causes the electrons to be more closely associated with the oxygen atom, resulting in a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This charge separation creates a permanent dipole moment for the water molecule.
Other molecules, such as carbon dioxide (CO₂), possess symmetrical structures that cancel out individual bond dipoles, leading to a net zero dipole moment despite having polar bonds. This demonstrates that the molecular geometry plays a critical role in determining the overall dipole moment.
Influence of Dipoles on Molecular Interactions
The presence of dipole moments significantly influences intermolecular forces, affecting the physical and chemical properties of substances. Dipole-dipole interactions, where the positive end of one dipole attracts the negative end of another, are a crucial factor in determining melting points, boiling points, and solubility. The stronger the dipole moment, the stronger these interactions.
Hydrogen bonding, a particularly strong type of dipole-dipole interaction involving hydrogen bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine), significantly impacts the properties of many biological molecules, including proteins and DNA.
Applications of Dipoles: From Technology to Biology
Dipoles are not just theoretical constructs; they have practical applications across a broad range of disciplines:
1. Capacitors: Storing Electrical Energy
Capacitors, essential components in electronic circuits, rely on the principle of storing energy in an electric field created between two oppositely charged plates separated by a dielectric material. The effectiveness of a capacitor is directly related to the capacitance, which depends on the area of the plates and the distance between them. The electric field between the plates resembles the field of a parallel plate capacitor, which can be approximated by a dipole field in certain configurations.
2. Antennas: Radiating Electromagnetic Waves
Antennas, used for transmitting and receiving radio waves, operate by generating or detecting oscillating dipole moments. The alternating current flowing in the antenna creates an oscillating electric dipole moment, radiating electromagnetic waves into space. The design and geometry of antennas are tailored to optimize the radiation pattern and efficiency.
3. Molecular Spectroscopy: Studying Molecular Structure
Dipoles play a crucial role in various spectroscopic techniques used to study the structure and properties of molecules. Techniques like microwave spectroscopy and infrared spectroscopy exploit the interaction of electromagnetic radiation with the dipole moments of molecules, providing valuable insights into their vibrational and rotational modes.
4. Materials Science: Designing Novel Materials
The understanding and manipulation of dipole moments are essential in materials science for designing novel materials with specific electrical, optical, and magnetic properties. Materials with large dipole moments are used in various applications, including piezoelectric materials (converting mechanical stress into electrical energy) and ferroelectric materials (possessing spontaneous electric polarization).
Beyond the Basic Dipole: Higher-Order Multipoles
While the simple dipole model is remarkably useful, it's important to acknowledge that real-world charge distributions are often more complex than two point charges. In such cases, higher-order multipoles, such as quadrupoles (four charges arranged in a specific pattern) and octupoles (eight charges), are necessary for accurate representation of the electric field. These higher-order multipoles contribute to finer details of the electric field distribution and play a crucial role in sophisticated analyses.
Conclusion: The Enduring Significance of Dipoles
The concept of two opposite charges separated by a small distance, though seemingly simple, is fundamental to our understanding of electromagnetism and its impact on the physical world. From the behavior of molecules to the design of advanced technologies, dipoles play a crucial and pervasive role. This article has explored the fundamental properties of dipoles, their applications, and their significance in diverse fields. The continued investigation and manipulation of dipoles will undoubtedly lead to further breakthroughs in science and technology. Further research into the complexities of dipole interactions and their applications continues to unveil a deeper understanding of the electromagnetic world around us. The study of dipoles remains a vibrant area of research with ongoing implications for various fields.
Latest Posts
Latest Posts
-
Every Computer Has An Operating System
Mar 25, 2025
-
Which Of The Following Is A Browser
Mar 25, 2025
-
What Is The Conjugate Base Of Nh4
Mar 25, 2025
-
How Many Years Did Rip Van Winkle Sleep
Mar 25, 2025
-
Number Of Valence Electrons In Calcium
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Two Opposite Charges Separated By A Small Distance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
