What Is The Conjugate Base Of Nh4+
News Leon
Mar 25, 2025 · 5 min read
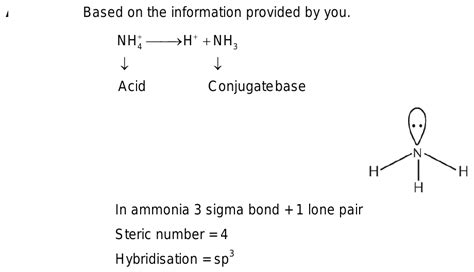
Table of Contents
What is the Conjugate Base of NH₄⁺? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on the conjugate base of the ammonium ion (NH₄⁺). We'll explore its formation, properties, and importance in various chemical processes.
Understanding Conjugate Acid-Base Pairs
According to the Brønsted-Lowry theory of acids and bases, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are always related by the difference of a single proton.
Think of it like this: an acid loses a proton to become its conjugate base, and a base gains a proton to become its conjugate acid. They are two sides of the same coin, differing only by the presence or absence of a proton (H⁺).
Identifying the Conjugate Base of NH₄⁺
The ammonium ion (NH₄⁺) is a weak acid. This means it doesn't completely dissociate in water, only partially donating its proton. When NH₄⁺ acts as an acid and donates a proton, it forms its conjugate base: ammonia (NH₃).
The reaction can be represented as follows:
NH₄⁺ ⇌ NH₃ + H⁺
In this equilibrium reaction, NH₄⁺ is the acid, donating a proton (H⁺) to become NH₃, its conjugate base. The equilibrium lies to the left, indicating that NH₄⁺ is a weak acid and does not readily donate its proton.
Properties of Ammonia (NH₃), the Conjugate Base
Ammonia (NH₃), the conjugate base of NH₄⁺, is a colorless gas with a pungent, characteristic odor. Its properties are significantly different from its conjugate acid:
1. Basicity:
Unlike its acidic conjugate, ammonia is a weak base. This means it partially accepts protons from water, forming hydroxide ions (OH⁻) and increasing the pH of the solution. The reaction in water is:
NH₃ + H₂O ⇌ NH₄⁺ + OH⁻
The equilibrium constant for this reaction, Kb, is a measure of the base's strength. A small Kb value, as seen with ammonia, indicates it's a weak base.
2. Lewis Base:
Ammonia is also a Lewis base. This is because it possesses a lone pair of electrons on the nitrogen atom, which can be donated to an electron-deficient species (a Lewis acid). This ability to donate electrons makes ammonia a nucleophile in many organic reactions.
3. Reactivity:
Ammonia's reactivity stems from its lone pair of electrons and its ability to act as both a Brønsted-Lowry base and a Lewis base. It reacts with many acids to form ammonium salts. It also reacts with various metal ions to form coordination complexes. Furthermore, it plays a crucial role in the synthesis of numerous nitrogen-containing compounds, including fertilizers and explosives.
4. Solubility:
Ammonia is highly soluble in water, a property exploited in various industrial and laboratory applications. Its solubility arises from hydrogen bonding between the ammonia molecule and water molecules.
Importance of the NH₄⁺/NH₃ Conjugate Pair
The NH₄⁺/NH₃ conjugate pair plays a significant role in numerous chemical and biological systems:
1. Buffer Solutions:
Mixtures of a weak acid and its conjugate base (or a weak base and its conjugate acid) form buffer solutions. These solutions resist changes in pH upon the addition of small amounts of acid or base. A mixture of NH₄Cl (which provides NH₄⁺) and NH₃ forms an effective buffer solution, commonly used in laboratories and industrial processes to maintain a relatively constant pH.
2. Biological Systems:
Ammonium ions (NH₄⁺) and ammonia (NH₃) are crucial in biological systems. Ammonium ions are a vital component of nitrogen metabolism in plants and animals. Plants absorb ammonium ions from the soil as a source of nitrogen, which is essential for building proteins and nucleic acids. In animals, excess nitrogen is excreted as urea, a product of ammonia metabolism. The equilibrium between NH₄⁺ and NH₃ is critical in maintaining the acid-base balance within biological systems.
3. Industrial Applications:
Ammonia is a cornerstone chemical in numerous industrial processes. Its large-scale production via the Haber-Bosch process is a cornerstone of modern agriculture, as ammonia serves as a precursor for fertilizers. It also finds applications in the production of various chemicals, explosives, and pharmaceuticals. The acidity and basicity of NH₄⁺ and NH₃, respectively, play important roles in controlling reaction conditions and outcomes in these processes.
4. Water Treatment:
Ammonium ions can be present in wastewater as a result of decomposition of organic matter. In water treatment processes, the conversion of ammonium to nitrate through nitrification (a process involving several bacteria) is critical for removing this nitrogen source and preventing eutrophication. Understanding the acid-base chemistry of NH₄⁺ is essential for effectively optimizing this process.
Distinguishing NH₄⁺ and NH₃
It's crucial to clearly distinguish between NH₄⁺ and NH₃, given their contrasting properties:
| Feature | NH₄⁺ (Ammonium Ion) | NH₃ (Ammonia) |
|---|---|---|
| Charge | +1 | 0 |
| Acid/Base | Weak acid | Weak base |
| Structure | Tetrahedral | Trigonal pyramidal |
| Solubility in Water | Highly soluble | Highly soluble |
| Reactivity | Reacts with bases | Reacts with acids |
| Biological Role | Nitrogen source | Nitrogen metabolism |
Conclusion: The Significance of Conjugate Pairs
The ammonium ion (NH₄⁺) and its conjugate base, ammonia (NH₃), exemplify the crucial concept of conjugate acid-base pairs. Understanding their properties, interconversion, and roles in various systems—from buffer solutions to biological processes and industrial applications—is critical in chemistry. Their importance underscores the fundamental principles of acid-base chemistry and its far-reaching implications in numerous scientific fields. The equilibrium between NH₄⁺ and NH₃ is dynamically influenced by pH and plays a vital role in maintaining chemical and biological homeostasis. Further exploration of this fascinating conjugate pair reveals ever-deeper connections to a wide array of scientific and technological advances. The versatility and ubiquity of ammonia and the ammonium ion cement their significance in the world of chemistry and beyond. Their ongoing study remains a cornerstone of chemical research and innovation.
Latest Posts
Latest Posts
-
Words Beginning With The Same Letter
Mar 26, 2025
-
The Proper Order For The Scientific Process Is
Mar 26, 2025
-
What Is The Lightest Element On The Periodic Table
Mar 26, 2025
-
Which Of The Following Represent Statistical Information
Mar 26, 2025
-
Which Of The Following Is A Steroid Hormone
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Base Of Nh4+ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
