Number Of Valence Electrons In Calcium
News Leon
Mar 25, 2025 · 6 min read
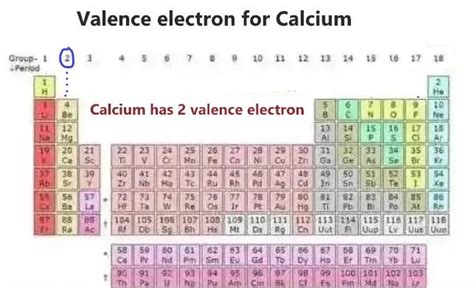
Table of Contents
- Number Of Valence Electrons In Calcium
- Table of Contents
- Unveiling the Secrets of Calcium: A Deep Dive into its Valence Electrons
- What are Valence Electrons?
- Determining Calcium's Valence Electrons: The Electronic Configuration
- Calcium's Reactivity: The Influence of Valence Electrons
- Chemical Bonding in Calcium Compounds: Ionic Bonds
- Biological Significance: Calcium's Role in Living Organisms
- Comparison with Other Group 2 Elements: Trends in Valence Electrons
- Beyond the Basics: Advanced Concepts Related to Calcium's Valence Electrons
- Conclusion: The Significance of Understanding Calcium's Valence Electrons
- Latest Posts
- Latest Posts
- Related Post
Unveiling the Secrets of Calcium: A Deep Dive into its Valence Electrons
Calcium, a vital element for life, plays a crucial role in numerous biological processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and biological significance. This comprehensive article will explore the intricacies of calcium's valence electrons, delving into its atomic structure, bonding characteristics, and the implications for its reactivity.
What are Valence Electrons?
Before diving into the specifics of calcium, let's establish a clear understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of compounds it can form. They dictate how an atom interacts with other atoms, leading to the formation of molecules and ionic compounds. The number of valence electrons is crucial for predicting an element's chemical properties.
Determining Calcium's Valence Electrons: The Electronic Configuration
To determine the number of valence electrons in calcium (Ca), we need to examine its electronic configuration. This configuration describes how electrons are distributed among the various energy levels and sublevels within the atom. Calcium's atomic number is 20, meaning it possesses 20 protons and 20 electrons in a neutral atom.
The electronic configuration of calcium is 1s²2s²2p⁶3s²3p⁶4s². Let's break this down:
- 1s²: Two electrons occupy the first energy level (n=1), specifically the s subshell.
- 2s²2p⁶: Eight electrons occupy the second energy level (n=2), with two in the s subshell and six in the p subshell.
- 3s²3p⁶: Eight electrons occupy the third energy level (n=3), with two in the s subshell and six in the p subshell.
- 4s²: Two electrons occupy the fourth energy level (n=4), specifically the s subshell.
The outermost shell of calcium is the fourth energy level (n=4), which contains two electrons in the 4s subshell. Therefore, calcium has 2 valence electrons.
Calcium's Reactivity: The Influence of Valence Electrons
The presence of only two valence electrons significantly influences calcium's reactivity. Elements strive for a stable electron configuration, often resembling that of a noble gas (a group 18 element). This stable configuration, characterized by a full outermost electron shell, is achieved through chemical bonding.
Calcium readily loses its two valence electrons to achieve a stable configuration similar to argon (Ar), a noble gas with 18 electrons. This loss of electrons results in the formation of a Ca²⁺ ion, a positively charged calcium ion. This process is known as ionization. The ease with which calcium loses its valence electrons explains its high reactivity, especially with nonmetals.
Chemical Bonding in Calcium Compounds: Ionic Bonds
Calcium's tendency to lose its two valence electrons leads primarily to the formation of ionic bonds. Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. When calcium reacts with a nonmetal, such as chlorine (Cl), calcium loses its two valence electrons to form the Ca²⁺ ion, while chlorine gains one electron to form the Cl⁻ ion. The resulting electrostatic attraction between the Ca²⁺ and Cl⁻ ions forms the ionic compound calcium chloride (CaCl₂).
The strength of the ionic bond depends on the charge of the ions and the distance between them. Because calcium forms a +2 ion, it forms strong ionic bonds with many nonmetals. This explains the high melting and boiling points observed in many calcium compounds.
Biological Significance: Calcium's Role in Living Organisms
Calcium's two valence electrons and its ability to form stable ionic bonds have profound implications for its biological significance. Calcium ions (Ca²⁺) play crucial roles in various biological processes, including:
-
Bone and tooth structure: Calcium is a major component of bones and teeth, providing structural support and strength. It forms strong ionic bonds with phosphate ions to create the hard mineral hydroxyapatite.
-
Muscle contraction: Calcium ions regulate muscle contraction by binding to proteins in muscle cells, triggering the sliding filament mechanism that leads to muscle movement.
-
Nerve impulse transmission: Calcium ions are essential for nerve impulse transmission, playing a role in the release of neurotransmitters at nerve synapses.
-
Blood clotting: Calcium ions are involved in the complex cascade of events leading to blood clotting, preventing excessive bleeding.
-
Enzyme activation: Many enzymes require calcium ions for their proper function, acting as cofactors that help the enzyme catalyze biological reactions.
Comparison with Other Group 2 Elements: Trends in Valence Electrons
Calcium belongs to Group 2 of the periodic table, also known as the alkaline earth metals. All elements in this group have two valence electrons, sharing similar chemical properties and reactivity. However, there are subtle differences in their reactivity due to variations in atomic size and ionization energy.
For example, magnesium (Mg), located above calcium in Group 2, has a smaller atomic radius and higher ionization energy than calcium. This means magnesium holds onto its two valence electrons more tightly than calcium, making it slightly less reactive. Conversely, strontium (Sr) and barium (Ba), located below calcium, have larger atomic radii and lower ionization energies, making them more reactive than calcium. The trend in reactivity within Group 2 is directly related to the ease with which the two valence electrons are lost.
Beyond the Basics: Advanced Concepts Related to Calcium's Valence Electrons
The concept of valence electrons extends beyond the simple counting of electrons in the outermost shell. More advanced concepts help us to understand calcium's behavior in more complex scenarios:
-
Oxidation state: Calcium almost always exhibits an oxidation state of +2, reflecting the loss of its two valence electrons during chemical reactions. This predictable oxidation state simplifies the prediction of its chemical behavior.
-
Coordination chemistry: Calcium ions can form coordination complexes with various ligands (molecules or ions that bind to the metal ion). The number of ligands that can bind to a Ca²⁺ ion is determined by its coordination number, influenced by the size and charge of the ion and the nature of the ligands.
-
Spectroscopy: Spectroscopic techniques, such as atomic absorption spectroscopy and flame emission spectroscopy, can be used to quantify the concentration of calcium ions in various samples. These techniques exploit the unique electronic transitions of calcium atoms and ions.
-
Quantum mechanics: A deeper understanding of the distribution of valence electrons in calcium requires the application of quantum mechanics, providing a more precise description of electron orbitals and their energies.
Conclusion: The Significance of Understanding Calcium's Valence Electrons
Understanding the number of valence electrons in calcium—two—is fundamental to comprehending its chemical behavior, reactivity, and biological significance. This knowledge allows us to predict the types of compounds calcium forms, its role in biological processes, and its interactions with other elements. The information presented here provides a solid foundation for further exploration into the fascinating world of calcium chemistry and its multifaceted roles in various scientific disciplines. From the formation of strong ionic bonds to its vital role in living organisms, calcium's two valence electrons are central to its importance in the natural world. This deep dive into calcium’s valence electrons showcases the power of fundamental chemistry principles in understanding the macroscopic world around us.
Latest Posts
Latest Posts
-
Which Of The Following Reactions Is Not Reversible
Mar 27, 2025
-
How To Find Moles Of An Element
Mar 27, 2025
-
All The Different Populations That Live Together In An Area
Mar 27, 2025
-
What Is Another Name For Angle 1
Mar 27, 2025
-
Is Magnetizing Steel A Chemical Change
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Calcium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
