Is Magnetizing Steel A Chemical Change
News Leon
Mar 27, 2025 · 5 min read
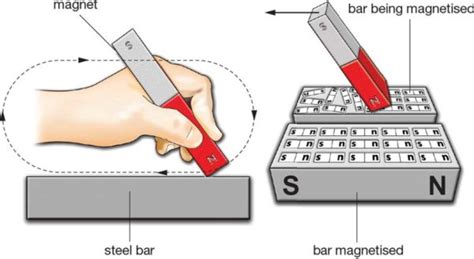
Table of Contents
Is Magnetizing Steel a Chemical Change? A Deep Dive into Physical vs. Chemical Transformations
The question of whether magnetizing steel constitutes a chemical change is a classic example of the subtle differences between physical and chemical transformations. While seemingly simple, understanding this distinction requires a deeper look into the atomic structure of matter and the nature of magnetism. This article will explore the intricacies of this question, examining the processes involved and clarifying the key differences between physical and chemical changes.
Understanding Chemical and Physical Changes
Before delving into the specifics of magnetizing steel, let's establish a clear definition of chemical and physical changes.
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves a fundamental alteration in the chemical composition of a substance. This means that the atoms within the substance rearrange themselves to form new molecules with different properties. Classic indicators of a chemical change include:
- Formation of a new substance: The original substance is transformed into a completely different substance with distinct characteristics.
- Change in color: A noticeable color shift often signals a chemical reaction.
- Evolution of gas: The production of bubbles or gas indicates the formation of new gaseous products.
- Formation of a precipitate: The formation of a solid from a solution is another sign of a chemical reaction.
- Release or absorption of heat: Chemical reactions are either exothermic (release heat) or endothermic (absorb heat).
Physical Changes: Altering Appearance, Not Composition
A physical change, on the other hand, only affects the physical properties of a substance without altering its chemical composition. The substance retains its fundamental identity, even though its form or appearance might change. Examples include:
- Changes in state: Melting, freezing, boiling, and condensation are all physical changes.
- Changes in shape: Bending, cutting, or crushing a material are physical changes.
- Dissolving: Dissolving salt in water is a physical change; the salt molecules are still present, just dispersed in the water.
- Magnetization (in most cases): This is where the crux of our discussion lies.
The Nature of Magnetism in Steel
To understand the effect of magnetization on steel, we need to explore the microscopic world of ferromagnetism. Steel, an alloy primarily composed of iron, is a ferromagnetic material. This means it possesses strong magnetic properties due to the arrangement of its electrons.
Electron Spin and Magnetic Domains
At the atomic level, electrons possess an intrinsic property called spin, which creates a tiny magnetic field. In most materials, these electron spins are randomly oriented, canceling out their magnetic effects. However, in ferromagnetic materials like iron, a significant number of electron spins align parallel to each other within regions called magnetic domains.
Each domain acts like a tiny magnet. In an unmagnetized piece of steel, these domains are randomly oriented, resulting in no overall magnetic field.
The Magnetization Process: Aligning Domains
Magnetizing steel involves aligning these magnetic domains. This is achieved by applying an external magnetic field. The field exerts a force on the domains, causing them to rotate and align themselves with the field. Once the external field is removed, many of these domains remain aligned, giving the steel a net magnetic moment. This is why the steel becomes a permanent magnet.
Is Magnetization a Chemical or Physical Change?
Now, let's return to the central question: Is magnetizing steel a chemical change? The answer is unequivocally no. Magnetizing steel is a physical change.
Here's why:
- No new substance is formed: The chemical composition of the steel remains unchanged. It's still primarily iron and carbon (or other alloying elements). The only change is the arrangement of the magnetic domains within the material.
- The change is reversible (to some extent): The magnetization can be reduced or even completely reversed by applying a strong opposing magnetic field or by heating the steel above its Curie temperature. This is characteristic of a physical change. A chemical change is typically irreversible without further chemical intervention.
- No bonds are broken or formed: The chemical bonds between the iron atoms and other elements within the steel remain intact throughout the magnetization process. Only the orientation of magnetic domains changes.
Distinguishing Factors: A Comparative Table
To further solidify the understanding, let's compare magnetizing steel to a definitive chemical change, such as rusting:
| Feature | Magnetizing Steel (Physical Change) | Rusting Steel (Chemical Change) |
|---|---|---|
| Chemical Composition | Unchanged | Changed (formation of iron oxides) |
| Bonding | No bonds broken or formed | Bonds broken and reformed |
| Reversibility | Largely reversible | Irreversible |
| New Substance Formed | No | Yes (iron oxides) |
| External Factors | External magnetic field | Exposure to oxygen and water |
| Microscopic Changes | Domain alignment | Atomic rearrangement |
Other Relevant Concepts
Several related concepts can further illuminate the distinction:
Curie Temperature
The Curie temperature is the temperature above which a ferromagnetic material loses its permanent magnetism. Above this temperature, the thermal energy overcomes the forces that align the magnetic domains, resulting in random orientation and the loss of magnetization. This is a physical change, not a chemical one.
Magnetic Hysteresis
Magnetic hysteresis refers to the relationship between the magnetic field applied to a material and the resulting magnetization. The magnetization doesn't instantly respond to the applied field; instead, there's a lag, resulting in a hysteresis loop. This loop illustrates the reversibility of magnetization, again supporting its physical nature.
Types of Magnetism
Understanding the different types of magnetism, such as diamagnetism, paramagnetism, and ferromagnetism, provides a broader context for understanding the behavior of materials under magnetic fields. Ferromagnetism, with its domain structure and ability to retain magnetization, is unique and vital in the context of our discussion.
Conclusion: Magnetization—A Physical Transformation
In conclusion, magnetizing steel is undeniably a physical change, not a chemical one. No new substance is formed, the chemical composition remains unaltered, and the change is largely reversible. The process involves the manipulation of existing magnetic domains within the material, a phenomenon occurring without breaking or forming chemical bonds. Understanding this distinction highlights the crucial differences between physical and chemical transformations and underscores the fascinating interplay between macroscopic properties and microscopic atomic structure. This knowledge is essential for understanding a wide range of materials science applications, from magnetic storage devices to advanced materials engineering.
Latest Posts
Latest Posts
-
What Is The Oxidation State Of Nitrogen In Nano2
Mar 30, 2025
-
Is Paper A Conductor Or Insulator
Mar 30, 2025
-
Draw The Major Product S Of The Following Reaction
Mar 30, 2025
-
Oxidation Number Of P In Po43
Mar 30, 2025
-
What Are The Raw Materials For Cellular Respiration
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Magnetizing Steel A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
