Draw The Major Product S Of The Following Reaction
News Leon
Mar 30, 2025 · 6 min read
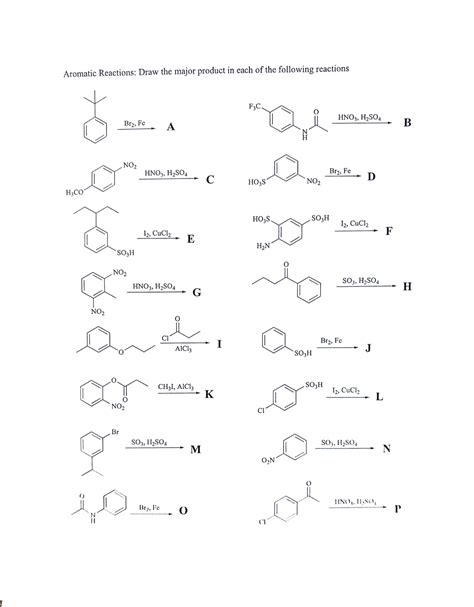
Table of Contents
Drawing the Major Products of Organic Reactions: A Comprehensive Guide
Predicting the major product of an organic reaction is a cornerstone of organic chemistry. It requires a thorough understanding of reaction mechanisms, functional group transformations, and the interplay of various factors influencing reaction pathways. This article will delve into the principles governing product prediction, offering a detailed approach to tackling diverse reaction scenarios. We'll explore several reaction types, providing step-by-step analyses and highlighting key considerations for determining the major product.
Understanding Reaction Mechanisms: The Foundation of Product Prediction
Before predicting products, a firm grasp of the reaction mechanism is crucial. The mechanism outlines the step-by-step process of bond breaking and bond formation, revealing the intermediates and transition states involved. This understanding allows us to anticipate which pathways are energetically more favorable and, therefore, more likely to lead to the major product. Key aspects to consider include:
1. Nucleophilic and Electrophilic Reactions:
Nucleophilic reactions involve a nucleophile (electron-rich species) attacking an electrophile (electron-deficient species). The nucleophile donates an electron pair to the electrophile, forming a new bond. The leaving group, if present, departs.
Electrophilic reactions involve an electrophile attacking a nucleophile. The electrophile accepts an electron pair from the nucleophile.
Understanding the nature of the reactants (nucleophile or electrophile) is key to determining the site of attack and the subsequent product formation.
2. Stereochemistry:
Stereochemistry plays a crucial role in predicting products. Consider the following:
- Chirality: Reactions can create or destroy chiral centers. Understanding stereoselectivity (preference for one stereoisomer over another) is essential.
- Regioselectivity: In reactions involving multiple possible sites of attack, regioselectivity dictates which site is preferentially attacked. Markovnikov's rule and anti-Markovnikov's rule are examples of regioselectivity principles.
- Stereospecificity: A stereospecific reaction yields specific stereoisomers depending on the configuration of the starting material.
3. Kinetic vs. Thermodynamic Control:
Reactions can be under kinetic or thermodynamic control.
- Kinetic control: The major product is the one formed faster, often at lower temperatures. This is governed by the activation energy of the reaction pathway.
- Thermodynamic control: The major product is the most stable product, often favored at higher temperatures. This is determined by the relative Gibbs free energy of the products.
Illustrative Examples: Predicting Major Products
Let's examine several reaction types and illustrate how to predict their major products:
1. SN1 and SN2 Reactions:
SN1 (Substitution Nucleophilic Unimolecular) reactions: These involve a two-step mechanism: ionization to form a carbocation intermediate, followed by nucleophilic attack. The rate-determining step is the ionization, making the stability of the carbocation crucial. More substituted carbocations are more stable (tertiary > secondary > primary). Therefore, SN1 reactions favor the formation of the most stable carbocation intermediate, leading to a specific product.
SN2 (Substitution Nucleophilic Bimolecular) reactions: These are concerted reactions, meaning bond breaking and bond formation occur simultaneously. The reaction proceeds via backside attack by the nucleophile, leading to inversion of configuration at the stereocenter. Steric hindrance plays a significant role; less hindered substrates react faster.
Example: Consider the reaction of 2-bromobutane with sodium methoxide (NaOCH₃). In polar aprotic solvents, this favors an SN2 reaction, leading to inversion of configuration at the chiral carbon. The major product will be (S)-2-methoxybutane if the starting material was (R)-2-bromobutane.
2. Electrophilic Addition Reactions:
These reactions involve the addition of an electrophile to a double or triple bond. Markovnikov's rule often governs the regioselectivity, predicting that the electrophile adds to the carbon atom with the fewest hydrogen atoms. However, anti-Markovnikov addition can occur in the presence of specific reagents like peroxides.
Example: The addition of HBr to propene. Markovnikov's rule predicts the major product to be 2-bromopropane, with the Br atom attaching to the more substituted carbon.
3. Elimination Reactions:
Elimination reactions involve the removal of atoms or groups from a molecule, often forming a double or triple bond. Two common types are E1 and E2.
E1 (Elimination Unimolecular) reactions: These involve a two-step mechanism: formation of a carbocation intermediate, followed by base abstraction of a proton. The stability of the carbocation governs the regioselectivity, favoring the formation of the more substituted alkene (Zaitsev's rule).
E2 (Elimination Bimolecular) reactions: These are concerted reactions where proton abstraction and bond breaking occur simultaneously. The stereochemistry is crucial; anti-periplanar geometry is preferred for the departing groups. Zaitsev's rule often dictates the regioselectivity.
Example: Dehydration of 2-butanol. The E1 mechanism (acid-catalyzed) favors the formation of the more substituted alkene, 2-butene, while the E2 mechanism (strong base) can lead to a mixture of 2-butene and 1-butene depending on reaction conditions and base choice.
4. Friedel-Crafts Reactions:
These reactions involve the electrophilic aromatic substitution of an alkyl or acyl group onto an aromatic ring. The electrophile attacks the aromatic ring, forming a sigma complex, which then loses a proton to regenerate the aromatic ring. The reaction is subject to steric and electronic effects.
Example: Friedel-Crafts alkylation of benzene with chloromethane in the presence of aluminum chloride (AlCl₃). The major product is toluene (methylbenzene).
5. Oxidation and Reduction Reactions:
These reactions involve the gain or loss of electrons. Predicting the products requires understanding the oxidizing or reducing agent's strength and the susceptibility of specific functional groups to oxidation or reduction.
Example: Oxidation of a primary alcohol using potassium permanganate (KMnO₄). The major product will be a carboxylic acid.
Advanced Considerations in Predicting Major Products
Several factors can influence the major product beyond the fundamental principles discussed above:
- Solvent Effects: The solvent can significantly impact reaction rates and selectivity. Polar protic solvents favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 and E2 reactions.
- Temperature: As mentioned, temperature influences whether the reaction is kinetically or thermodynamically controlled.
- Catalyst: Catalysts can alter reaction pathways and influence product distribution.
- Concentration of Reactants: The concentration of reactants can affect the relative rates of competing reactions.
- Steric Hindrance: Bulky groups can hinder the approach of reactants, affecting reaction rates and selectivity.
Conclusion: Mastering Product Prediction
Predicting the major product of an organic reaction requires a systematic approach. This involves a thorough understanding of reaction mechanisms, stereochemistry, and the influence of various factors affecting reaction pathways. By diligently applying these principles and carefully considering the specific reaction conditions, one can confidently predict the major product in a wide array of organic transformations. Remember to always analyze the reactants, the reaction conditions, and the potential mechanisms to arrive at the most likely outcome. Consistent practice and a strong foundational understanding of organic chemistry principles are key to mastering this critical skill. Continue practicing diverse reactions, and you will progressively enhance your ability to accurately predict the major products of organic reactions.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Monomial
Apr 01, 2025
-
Which Of The Following Is Not A Lymphatic Organ
Apr 01, 2025
-
An Astronauts Weight On Earth Is 800 N
Apr 01, 2025
-
What Is The Percent Of 13 20
Apr 01, 2025
-
If A Transversal Intersects Two Parallel Lines Then
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Product S Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
