How To Find Moles Of An Element
News Leon
Mar 27, 2025 · 5 min read
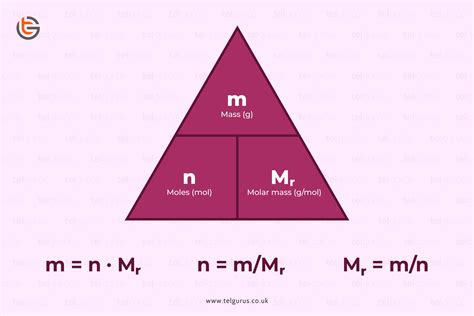
Table of Contents
How to Find Moles of an Element: A Comprehensive Guide
Determining the number of moles of an element is a fundamental concept in chemistry, crucial for various calculations and understanding chemical reactions. This comprehensive guide will walk you through different methods to calculate moles, focusing on clarity and practical application. We'll explore various scenarios and provide examples to solidify your understanding.
Understanding the Mole Concept
Before diving into calculations, let's establish a firm grasp of what a mole represents. A mole (mol) is a unit of measurement in chemistry that represents Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of particles. These particles can be atoms, molecules, ions, or even formula units, depending on the substance. Essentially, one mole of any substance contains the same number of particles as one mole of any other substance.
This concept is vital because it provides a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and liters that we can measure in a laboratory. It allows us to relate the mass of a substance to the number of atoms or molecules present.
Methods to Find Moles of an Element
The most common methods for calculating the number of moles of an element involve using its atomic mass and the mass of the sample. Let's examine these methods in detail.
1. Using Mass and Atomic Mass
This is the most straightforward method. You'll need two pieces of information:
- The mass of the element sample (in grams): This is the quantity you measure directly using a balance.
- The atomic mass of the element (in atomic mass units or amu): This value is found on the periodic table. It represents the average mass of all isotopes of the element, weighted by their natural abundance. Note that for simplicity, we often use the atomic mass as the molar mass (grams per mole).
The formula to calculate the number of moles (n) is:
n = mass (g) / molar mass (g/mol)
Example:
Let's calculate the number of moles in 10 grams of iron (Fe). The atomic mass of iron from the periodic table is approximately 55.85 g/mol.
n = 10 g / 55.85 g/mol n ≈ 0.179 moles
Therefore, 10 grams of iron contain approximately 0.179 moles of iron atoms.
2. Using Avogadro's Number and the Number of Atoms
If you know the number of atoms present in a sample, you can also calculate the number of moles using Avogadro's number:
n = Number of atoms / Avogadro's number
Example:
Suppose you have 3.011 x 10<sup>23</sup> atoms of gold (Au). To find the number of moles:
n = 3.011 x 10<sup>23</sup> atoms / 6.022 x 10<sup>23</sup> atoms/mol n ≈ 0.5 moles
This means you have 0.5 moles of gold atoms.
3. Dealing with Compounds: Finding Moles of an Element within a Compound
When dealing with compounds, things get slightly more complex. You need to consider the molar mass of the compound and the element's stoichiometry within the compound's formula.
Example:
Let's determine the moles of oxygen in 100 grams of water (H₂O).
-
Calculate the molar mass of water:
- H: 1.01 g/mol x 2 = 2.02 g/mol
- O: 16.00 g/mol
- Total molar mass of H₂O: 2.02 g/mol + 16.00 g/mol = 18.02 g/mol
-
Calculate the moles of water: n(H₂O) = 100 g / 18.02 g/mol ≈ 5.55 moles
-
Determine the moles of oxygen: There is one oxygen atom per water molecule. Therefore, the moles of oxygen are equal to the moles of water. n(O) = 5.55 moles
Therefore, there are approximately 5.55 moles of oxygen atoms in 100 grams of water.
More complex example: Consider finding the moles of iron in 50 grams of iron(III) oxide (Fe₂O₃).
-
Calculate the molar mass of Fe₂O₃:
- Fe: 55.85 g/mol x 2 = 111.7 g/mol
- O: 16.00 g/mol x 3 = 48.00 g/mol
- Total molar mass of Fe₂O₃: 111.7 g/mol + 48.00 g/mol = 159.7 g/mol
-
Calculate the moles of Fe₂O₃: n(Fe₂O₃) = 50 g / 159.7 g/mol ≈ 0.313 moles
-
Determine the moles of iron: There are two iron atoms per formula unit of Fe₂O₃. n(Fe) = 0.313 moles Fe₂O₃ x (2 moles Fe / 1 mole Fe₂O₃) ≈ 0.626 moles
Thus, there are approximately 0.626 moles of iron atoms in 50 grams of iron(III) oxide.
Practical Applications and Importance
Understanding how to calculate moles is fundamental to various aspects of chemistry and related fields:
-
Stoichiometry: This is the cornerstone of chemical calculations, allowing you to determine the quantities of reactants and products in chemical reactions. Accurate mole calculations are essential for predicting reaction yields and limiting reactants.
-
Solution Chemistry: Molarity (moles per liter) is a critical unit for expressing the concentration of solutions. Calculations involving molarity rely heavily on accurate mole determinations.
-
Gas Laws: The ideal gas law (PV = nRT) uses moles to relate the pressure, volume, and temperature of a gas.
-
Titrations: In analytical chemistry, titrations rely on precise mole calculations to determine the concentration of unknown solutions.
-
Material Science: Understanding the stoichiometry of materials is vital in fields like metallurgy and ceramics. Mole calculations allow for the precise control of material properties.
Troubleshooting and Common Mistakes
-
Using incorrect atomic masses: Always double-check the atomic mass on the periodic table. Using the wrong value will lead to significant errors.
-
Unit errors: Pay close attention to units. Make sure to use grams for mass and g/mol for molar mass. Inconsistencies in units can easily lead to incorrect results.
-
Stoichiometry in compounds: When dealing with compounds, carefully consider the number of atoms of each element in the chemical formula.
-
Significant figures: Report your answers with the appropriate number of significant figures. The precision of your calculations should reflect the precision of your measurements.
Conclusion
Calculating the number of moles of an element is a core skill in chemistry. Mastering this skill is essential for a thorough understanding of chemical concepts and their applications. By understanding the fundamental principles and practicing the methods outlined in this guide, you can confidently perform mole calculations in various contexts, contributing to your overall success in chemistry and related fields. Remember to always double-check your work, pay attention to detail, and utilize the periodic table as your trusted resource. Consistent practice will build your proficiency and confidence in solving mole-related problems.
Latest Posts
Latest Posts
-
How Many Protons Electrons And Neutrons Are In Sodium
Mar 30, 2025
-
Mass Of Hydrogen Atom In G
Mar 30, 2025
-
Do Alkaline Earth Metals Occur Freely In Nature
Mar 30, 2025
-
Air Is Good Conductor Of Heat
Mar 30, 2025
-
Lines Of Symmetry On An Octagon
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How To Find Moles Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
