How Many Protons Electrons And Neutrons Are In Sodium
News Leon
Mar 30, 2025 · 5 min read
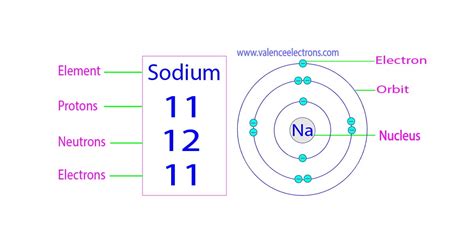
Table of Contents
How Many Protons, Electrons, and Neutrons are in Sodium? A Deep Dive into Atomic Structure
Sodium, a ubiquitous element crucial for life and numerous industrial applications, provides a fascinating case study for understanding atomic structure. This article will delve deep into the composition of a sodium atom, exploring the number of protons, electrons, and neutrons it contains, and explaining the concepts behind these subatomic particles. We will also touch upon isotopes and their implications.
Understanding Subatomic Particles: Protons, Electrons, and Neutrons
Before we delve into the specifics of sodium, let's establish a clear understanding of the three fundamental subatomic particles:
Protons: The Positive Charge Carriers
Protons are positively charged particles residing within the atom's nucleus. The number of protons in an atom's nucleus defines its atomic number and determines the element. This number is unique to each element and is found on the periodic table. A change in the number of protons fundamentally changes the element itself. For example, an atom with one proton is hydrogen, while an atom with two protons is helium.
Electrons: The Negative Charge Carriers
Electrons are negatively charged particles that orbit the nucleus in shells or energy levels. Unlike protons, the number of electrons in a neutral atom can vary without changing the element. Electrons are much lighter than protons and are responsible for chemical bonding and reactivity. They determine how an atom interacts with other atoms.
Neutrons: The Neutral Particles
Neutrons are neutrally charged particles, also located within the nucleus alongside protons. Unlike protons, the number of neutrons can vary without changing the element. However, changes in the neutron count result in different isotopes of the same element. Neutrons contribute significantly to the atom's mass.
Sodium's Atomic Structure: Unraveling the Numbers
Now, let's focus on sodium (Na), element number 11 on the periodic table. This means:
-
Number of Protons: Sodium possesses 11 protons in its nucleus. This defining characteristic makes it sodium and distinguishes it from all other elements.
-
Number of Electrons: In a neutral sodium atom, the number of electrons equals the number of protons. Therefore, a neutral sodium atom has 11 electrons. These electrons are arranged in specific energy levels or shells around the nucleus.
-
Number of Neutrons: This is slightly more complex. The number of neutrons is not fixed and can vary depending on the isotope. The most common isotope of sodium is Sodium-23 (²³Na). This notation indicates the mass number, which is the sum of protons and neutrons. Therefore, in Sodium-23:
Number of neutrons = Mass number - Number of protons = 23 - 11 = 12 neutrons
Isotopes: Variations in Neutron Count
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This means they have the same atomic number but different mass numbers. Sodium has several isotopes, but Sodium-23 is the most abundant, comprising over 99% of naturally occurring sodium. Other isotopes, like Sodium-22 and Sodium-24, exist but are less common and often radioactive.
Sodium-22 (²²Na)
This isotope has 11 protons and 11 neutrons (22 - 11 = 11). It is radioactive and decays through positron emission.
Sodium-24 (²⁴Na)
This isotope contains 11 protons and 13 neutrons (24 - 11 = 13). It is also radioactive and decays through beta decay. It has a relatively short half-life, making it useful in some medical applications like tracing blood flow.
The Significance of Sodium's Atomic Structure
The specific arrangement of protons, electrons, and neutrons in sodium dictates its physical and chemical properties. Its single electron in its outermost shell makes it highly reactive, readily losing this electron to form a +1 ion (Na⁺). This reactivity is fundamental to sodium's role in biological systems and various chemical processes.
Biological Importance
Sodium ions (Na⁺) play a vital role in various biological processes:
-
Nerve Impulse Transmission: Sodium channels in nerve cells are crucial for transmitting nerve impulses. The movement of sodium ions across cell membranes initiates action potentials.
-
Muscle Contraction: Sodium ions are essential for muscle contraction. Changes in sodium ion concentration trigger muscle fiber contraction.
-
Fluid Balance: Sodium helps regulate fluid balance within the body. It affects osmotic pressure and fluid distribution between cells and extracellular spaces.
Industrial Applications
Sodium's reactivity and properties lend themselves to various industrial applications:
-
Sodium Lamps: Sodium vapor lamps produce a characteristic yellow light, commonly used in street lighting due to their efficiency and brightness.
-
Sodium Chloride (Table Salt): Sodium chloride (NaCl), formed through the reaction of sodium with chlorine, is essential for human consumption and various industrial processes.
-
Sodium Hydroxide (Caustic Soda): Sodium hydroxide (NaOH) is a strong base used in various industries, including paper manufacturing, soap production, and water treatment.
Conclusion: A Comprehensive Understanding of Sodium's Composition
Understanding the number of protons, electrons, and neutrons in sodium is fundamental to comprehending its behavior and role in both biological systems and industrial processes. The 11 protons define it as sodium, while the 11 electrons in a neutral atom dictate its reactivity, and the variable neutron count explains the existence of different isotopes. Sodium-23, with its 12 neutrons, is the most prevalent isotope. Its unique atomic structure is responsible for its importance in biological functions like nerve impulse transmission and fluid balance, as well as its widespread industrial applications. This deep dive into sodium's atomic structure highlights the interconnectedness of atomic composition and macroscopic properties, showcasing the power of understanding fundamental scientific concepts. Further exploration into the quantum mechanical model of the atom would provide an even deeper understanding of electron configuration and chemical bonding within sodium.
Latest Posts
Latest Posts
-
An Astronauts Weight On Earth Is 800 N
Apr 01, 2025
-
What Is The Percent Of 13 20
Apr 01, 2025
-
If A Transversal Intersects Two Parallel Lines Then
Apr 01, 2025
-
State Any Two Effects Of Force
Apr 01, 2025
-
How Many Electrons Does Sodium Have In Its Outer Shell
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Electrons And Neutrons Are In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
